Volume 5, Issue 3 (2024)
J Clinic Care Skill 2024, 5(3): 111-116 |
Back to browse issues page
Article Type:
Subject:
Ethics code: IR.KAUMS.MEDNT.REC.1401.232
History
Received: 2024/06/10 | Accepted: 2024/08/16 | Published: 2024/09/8
Received: 2024/06/10 | Accepted: 2024/08/16 | Published: 2024/09/8
How to cite this article
Foroozanfard F, Gilasi H, Maboodi M, Taghavi S. Comparing the Rate of Chemical Pregnancy and Clinical Pregnancy after IUI Treatment According to Some Factors. J Clinic Care Skill 2024; 5 (3) :111-116
URL: http://jccs.yums.ac.ir/article-1-272-en.html
URL: http://jccs.yums.ac.ir/article-1-272-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Gametogenesis Research Center, Kashan University of Medical Sciences, Kashan, Iran
Full-Text (HTML) (1605 Views)
Introduction
Infertility is a reproductive system disorder defined as the failure to conceive after at least one year of regular, unprotected sexual intercourse [1- 3]. It affects approximately 10% of couples of reproductive age [4]. In developing countries, the prevalence of infertility is estimated to affect one in every four couples, and in some regions, such as South Asia, Central Asia, the Middle East, North Africa, and Central and Eastern Europe, the rate can reach as high as 30% [5]. About one-third of infertility cases have a male origin, one-third have a female origin, and the remaining third are due to a combination of both male and female factors [6, 7].
Among infertility treatments, intrauterine insemination (IUI) with or without ovulation stimulation is considered the first line of treatment due to its relatively low cost and non-invasive nature, with a fairly acceptable efficacy rate [8-11]. IUI is widely used for cervical factor infertility, male factor infertility, anovulation, endometriosis, and unexplained infertility [12]. The chance of spontaneous pregnancy in couples with unexplained infertility ranges from 1.3% to 4%, which increases to 10.5% to 17.9% with the aid of IUI [13]. In this procedure, prepared motile sperm are directly introduced into the uterine cavity using an insemination cannula [14, 15]. Various medications are used for ovulation stimulation, and stimulated cycles can increase the risk of multiple pregnancies. However, evidence suggests that the chance of live birth is higher in IUI cycles using ovulation-stimulating medications compared to those without [16-18]. Therefore, it is preferable to use ovulation-stimulating drugs that offer a higher live birth rate with a lower risk of multiple pregnancies while remaining cost-effective [19, 20].
The highest probability of successful IUI is observed in women under 30 years of age with cervical factor or anovulatory infertility, and when sperm motility exceeds 60%, with a sperm count greater than 4 million/mL [21]. Certain patient-related factors, such as fallopian tube disorders or male factor infertility, reduce the success rate of IUI. In such cases, it is recommended to use other assisted reproductive technologies (ART), such as in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) [22]. Several factors influence the success rate of IUI, including the cause and duration of infertility, the number of mature follicles, endometrial thickness, and sperm parameters [21-24]. Sperm count, motility, and morphology all impact the success of the procedure, with the best outcomes occurring when the total number of motile sperm exceeds approximately 10 million [25]. Evidence suggests that for unexplained infertility and endometriosis, IUI combined with controlled ovarian stimulation significantly improves pregnancy outcomes compared to IUI in natural cycles [26-28]. Although there is limited evidence regarding the efficacy of IUI for male factor infertility, if at least one million motile sperm are available for insemination, IUI can be considered as the first treatment [29, 30]. Previous studies have examined the impact of factors such as age [22], treatment regimens, causes of infertility [13], and sperm count [21] on the success of IUI individually. However, to date, no study has comprehensively analyzed all the factors influencing IUI treatment outcomes to identify those with the greatest impact. Therefore, the present study aims to determine the factors associated with biochemical and clinical pregnancy rates in women following IUI treatment over a five-year period.
Instrument & Methods
This was a cross-sectional study carried out at Kashan, Iran in 2023. The study population included patients diagnosed with infertility and treated with IUI at the Shahid Beheshti Infertility Center in Kashan between 22 November 2016 and 22 November 2021. The sample size was calculated using the specific formula for estimating a proportion in a finite population (α=0.05, maximum acceptable error=0.04, and pregnancy rate after IUI treatment from the study by Huang et al.=0.28 [23]). Based on these parameters, the required sample size was determined to be 473 for an infinite population and 465 for a finite population (N=789; equation 1). However, only 334 eligible cases were found in the records from the five-year period and were included in the study.
Equation 1.
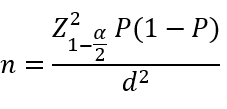
The inclusion criteria consisted of women between the ages of 18 and 40 who received IUI treatment from 22 November 2016 to 22 November 2021. The exclusion criterion was patients who had undergone ovulation stimulation using clomiphene citrate.
Data were collected using existing patient records, which were documented by the researcher in a data collection form. In this study, biochemical pregnancy was defined as a transient increase in β-hCG levels, and clinical pregnancy was determined by the presence of a gestational sac in ultrasound five weeks post-IUI.
When the proposal was approved and the ethics code obtained, eligible patient files from the records unit at Shahid Beheshti Infertility Center were reviewed. The researcher extracted data from these files into the study forms and then entered them into SPSS 16 software. The parameters collected included maternal age, body mass index (BMI), duration of infertility, male factor infertility, female factor infertility (endometriosis, diminished ovarian reserve (DOR), advanced age, polycystic ovarian syndrome (PCOS)), endometrial thickness (<8 mm, ≥8 mm), the number of mature follicles, the number of IUI cycles, and the type of ovulation-stimulating medication used.
SPSS was used for data analysis. Descriptive statistics were employed for quantitative parameters, including central tendency and dispersion indices. Frequency distributions were used to describe qualitative parameters. The absolute and relative frequencies of biochemical pregnancy and clinical pregnancy following IUI treatment were presented. Pregnancy and clinical pregnancy frequencies were also presented by background variables such as maternal age, BMI, and duration of infertility using cross-tabulations for qualitative parameters and means with standard deviations for quantitative parameters. Appropriate statistical tests were used for comparisons, including chi-square and Fisher's exact test for cross-tabulations, and an independent t-test for comparing quantitative parameters between two groups (α≤0.05).
Findings
In this study, 334 patients with infertility were examined. The average age of the patients in the study was 30.44±4.97 years. The mean Body Mass Index (BMI) of the patients was 26.66±4.19kg/m². The average number of IUI cycles among the patients was 1.67±0.76, and the average number of follicles during the last IUI cycle was 2.50±1.39 (Table 1).
Table 1. Demographic and Clinical Characteristics of the Studied Patients
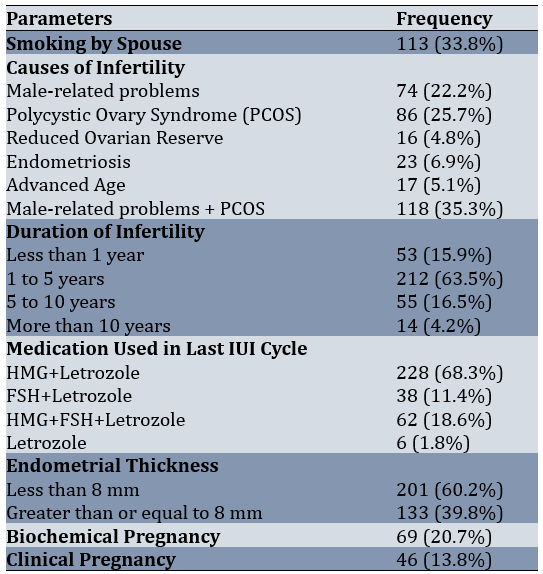
Patients underwent an average of 1.67±0.76 IUI cycles (with a minimum of 1 cycle and a maximum of 5 cycles). Additionally, they had an average of 2.50±1.39 follicles during the last IUI procedure (with a minimum of 1 follicle and a maximum of 7 follicles). Among the patients, 59.9% (n=201) had an endometrial thickness of less than 8 mm. Biochemical pregnancy occurred in 20.7% of the patients, while clinical pregnancy was observed in 13.8%.
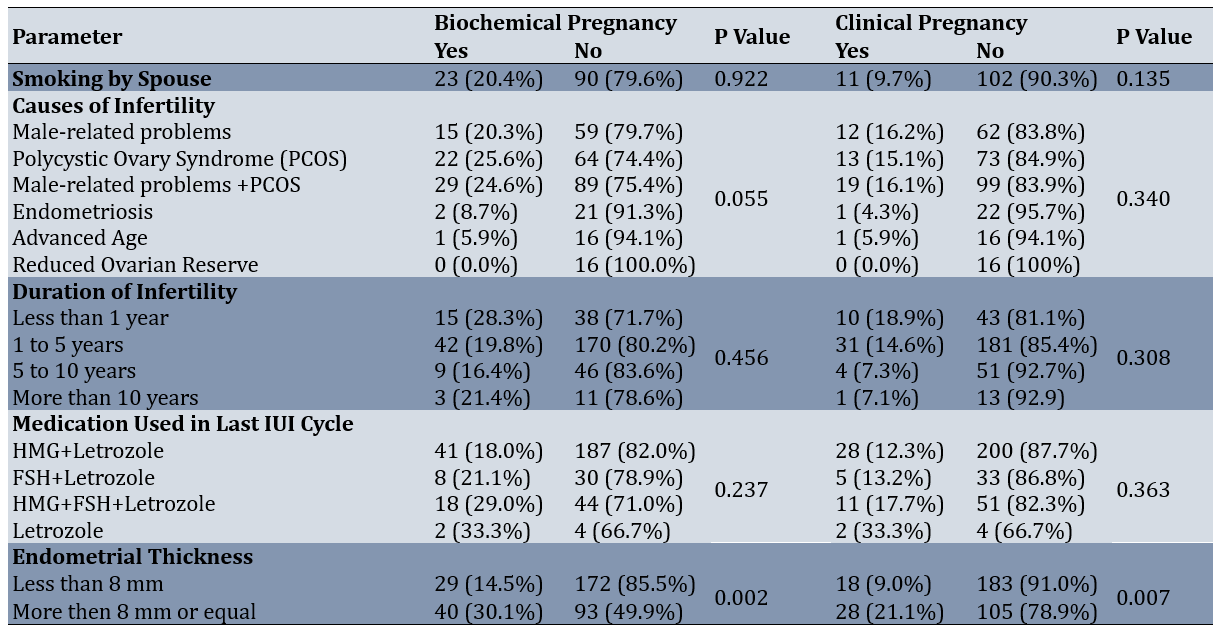
The average age of patients with positive biochemical pregnancy was 28.99±4.66 years, compared to 30.82±4.99 years for those with negative results. The average BMI was 26.84±4.77kg/m² for those with positive biochemical pregnancy and 26.62±4.03kg/m² for those with negative results. The average number of IUI cycles was 1.58±0.69 for positive cases and 1.69±0.78 for negative cases. The average number of follicles was 2.67±1.45 for patients with positive results and 2.46±1.38 for those with negative results. Regarding the comparison of parameters based on pregnancy outcomes, the mean age of patients with positive biochemical pregnancy results was significantly lower than those with negative results (p<0.006). Similarly, the mean age of patients with positive clinical pregnancy outcomes was significantly lower than those with negative outcomes (p<0.008). Moreover, a significant relationship was observed between biochemical pregnancy rates and endometrial thickness (p<0.002), indicating that patients with thicker endometrium responded better to treatment and had higher pregnancy rates. A significant association between clinical pregnancy rates and endometrial thickness was also found (p<0.007). Overall, younger patients and those with greater endometrial thickness experienced higher rates of both biochemical and clinical pregnancy (Table 2).
Table 2. Comparison of parameters based on biochemical and clinical pregnancy outcomes
Discussion
The aim of this study was to identify factors associated with biochemical and clinical pregnancy rates after IUI treatment over a 5-year period. Among infertility treatments, IUI, with or without ovulation stimulation, is the first-line therapy as it is relatively inexpensive, non-invasive, and has a reasonably acceptable efficacy [9-11]. In this study, the biochemical pregnancy rate was 20.7%, and the clinical pregnancy rate was 13.8%. The biochemical pregnancy rate per cycle was 12.2%, and the clinical pregnancy rate per cycle was 8.1%. Similar to our study, Craig et al. in the U.S. have analyzed 1959 IUI cycles in 661 women, reporting a pregnancy rate per cycle of 15.3% [31]. Additionally, Ahmed et al. in Oman have studied 227 women undergoing ovulation stimulation with clomiphene citrate and letrozole, with or without gonadotropins, and report an overall pregnancy rate of 21.58% [32]. These studies show pregnancy rates comparable to our findings.
BMI is an influential factor for pregnancy success and the effectiveness of IUI in ovulation stimulation cycles. The average BMI was 26.66 kg/m². However, BMI was not found to significantly affect IUI success. In contrast, Craig et al. report higher biochemical pregnancy rates in overweight and morbidly obese individuals compared to those with a normal BMI, and higher clinical pregnancy rates in overweight individuals compared to other BMI groups [31]. Similarly, Dodson et al. find out lower pregnancy rates in underweight women compared to normal and overweight women, despite adjusting for age and ovulation stimulation medication dosage. Although obese women require more medication and have lower E2 levels compared to overweight and normal-weight women, no significant difference in pregnancy rates among the BMI groups is observed, similar to our findings [33].
Female age is a crucial factor in pregnancy success and IUI outcomes. As age increases, pregnancy rates decrease. Our study confirmed this, showing a significant decrease in both biochemical and clinical pregnancy rates with increasing age. In line with this, Wang et al. have observed a noticeable decline in clinical pregnancy rates with age, with higher rates in women under 25 compared to those aged 25-30 and over 35 [34]. Likewise, Merviel et al. have analyzed 1038 IUI cycles in 353 couples and identify female age as the strongest factor influencing pregnancy rates, while infertility type and smoking status, as in our study, are not significant factors [22]. No significant relationship was found between the husband's smoking status and the biochemical or clinical pregnancy rates.
Infertility causes can affect IUI success. However, no significant relationship between different infertility causes and biochemical or clinical pregnancy rates was detected. The highest biochemical pregnancy rates were observed in patients with PCOS, while the highest clinical pregnancy rates were associated with male-factor infertility. Dickey et al., in a study of 8051 IUI cycles involving 4000 couples, also find out no significant relationship between infertility causes and pregnancy rates [35,36]. Auj and Farhamand's study show a decline in pregnancy rates with increasing age, although this is not statistically significant, and pregnancy rates are similar across different infertility causes [37].
The type of ovulation stimulation medication can influence IUI success. In this study, the type of medication did not significantly affect biochemical pregnancy rates (p=0.237) or clinical pregnancy rates (p=0.363). However, excluding the group treated only with letrozole, those receiving HMG+FSH in addition to letrozole had higher pregnancy and clinical pregnancy rates. Similarly, Diamond et al. show that clinical pregnancy rates in IUI cycles after ovulation stimulation with letrozole are significantly lower than with gonadotropins [38].
The duration of infertility can impact IUI success. In our study, no significant relationship was found between the duration of infertility and biochemical or clinical pregnancy rates. However, Huniadi et al. show that longer infertility duration reduces IUI success, with 80% of pregnancies occurring in cases of infertility lasting less than four years [39-40].
Endometrial thickness is another important factor in IUI success. Our study found a significant relationship between endometrial thickness and both biochemical and clinical pregnancy rates, with pregnancy rates increasing as endometrial thickness increased. Consistent with our results, Jayakrishnan et al. have studied 3851 IUI cycles in couples with male-factor infertility, anovulation, endometriosis, or unexplained infertility. They reported that IUI success increases with endometrial thickness, with the highest pregnancy rate (41%) observed at a thickness of 9-10 mm [41].
The number of IUI cycles and mature follicles per cycle can also affect IUI success. In the current study, as the number of cycles increased, pregnancy and clinical pregnancy rates decreased, but no significant relationship was found between the number of cycles and pregnancy rates. Similarly, no significant relationship was found between the number of follicles and pregnancy rates. Ahmed et al. also report a decrease in IUI success with an increasing number of cycles and no significant relationship between follicle number and biochemical or clinical pregnancy rates [32].
It should be noted that this study had some limitations. The BMI of the male partners was not recorded, which could have influenced IUI success. Some required information was not available in the patient records and was obtained via telephone interviews. Additionally, the number of eligible participants was smaller than the calculated sample size, which limited the study.
Given that age is a key factor affecting infertility, there is a clear need for education and counseling regarding earlier marriages and avoiding prolonged contraception. It is recommended that midwives and fertility counselors discuss the impact of age and endometrial thickness on the prognosis of IUI treatment with patients and suggest alternative advanced treatments if the prognosis is poor.
Conclusion
As the woman's age increases, the rates of pregnancy and clinical pregnancy decrease. Additionally, endometrial thickness significantly influences pregnancy and clinical pregnancy rates, with increased thickness leading to higher pregnancy and clinical pregnancy rates.
Infertility is a reproductive system disorder defined as the failure to conceive after at least one year of regular, unprotected sexual intercourse [1- 3]. It affects approximately 10% of couples of reproductive age [4]. In developing countries, the prevalence of infertility is estimated to affect one in every four couples, and in some regions, such as South Asia, Central Asia, the Middle East, North Africa, and Central and Eastern Europe, the rate can reach as high as 30% [5]. About one-third of infertility cases have a male origin, one-third have a female origin, and the remaining third are due to a combination of both male and female factors [6, 7].
Among infertility treatments, intrauterine insemination (IUI) with or without ovulation stimulation is considered the first line of treatment due to its relatively low cost and non-invasive nature, with a fairly acceptable efficacy rate [8-11]. IUI is widely used for cervical factor infertility, male factor infertility, anovulation, endometriosis, and unexplained infertility [12]. The chance of spontaneous pregnancy in couples with unexplained infertility ranges from 1.3% to 4%, which increases to 10.5% to 17.9% with the aid of IUI [13]. In this procedure, prepared motile sperm are directly introduced into the uterine cavity using an insemination cannula [14, 15]. Various medications are used for ovulation stimulation, and stimulated cycles can increase the risk of multiple pregnancies. However, evidence suggests that the chance of live birth is higher in IUI cycles using ovulation-stimulating medications compared to those without [16-18]. Therefore, it is preferable to use ovulation-stimulating drugs that offer a higher live birth rate with a lower risk of multiple pregnancies while remaining cost-effective [19, 20].
The highest probability of successful IUI is observed in women under 30 years of age with cervical factor or anovulatory infertility, and when sperm motility exceeds 60%, with a sperm count greater than 4 million/mL [21]. Certain patient-related factors, such as fallopian tube disorders or male factor infertility, reduce the success rate of IUI. In such cases, it is recommended to use other assisted reproductive technologies (ART), such as in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) [22]. Several factors influence the success rate of IUI, including the cause and duration of infertility, the number of mature follicles, endometrial thickness, and sperm parameters [21-24]. Sperm count, motility, and morphology all impact the success of the procedure, with the best outcomes occurring when the total number of motile sperm exceeds approximately 10 million [25]. Evidence suggests that for unexplained infertility and endometriosis, IUI combined with controlled ovarian stimulation significantly improves pregnancy outcomes compared to IUI in natural cycles [26-28]. Although there is limited evidence regarding the efficacy of IUI for male factor infertility, if at least one million motile sperm are available for insemination, IUI can be considered as the first treatment [29, 30]. Previous studies have examined the impact of factors such as age [22], treatment regimens, causes of infertility [13], and sperm count [21] on the success of IUI individually. However, to date, no study has comprehensively analyzed all the factors influencing IUI treatment outcomes to identify those with the greatest impact. Therefore, the present study aims to determine the factors associated with biochemical and clinical pregnancy rates in women following IUI treatment over a five-year period.
Instrument & Methods
This was a cross-sectional study carried out at Kashan, Iran in 2023. The study population included patients diagnosed with infertility and treated with IUI at the Shahid Beheshti Infertility Center in Kashan between 22 November 2016 and 22 November 2021. The sample size was calculated using the specific formula for estimating a proportion in a finite population (α=0.05, maximum acceptable error=0.04, and pregnancy rate after IUI treatment from the study by Huang et al.=0.28 [23]). Based on these parameters, the required sample size was determined to be 473 for an infinite population and 465 for a finite population (N=789; equation 1). However, only 334 eligible cases were found in the records from the five-year period and were included in the study.
Equation 1.
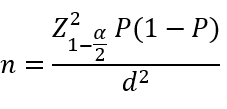
The inclusion criteria consisted of women between the ages of 18 and 40 who received IUI treatment from 22 November 2016 to 22 November 2021. The exclusion criterion was patients who had undergone ovulation stimulation using clomiphene citrate.
Data were collected using existing patient records, which were documented by the researcher in a data collection form. In this study, biochemical pregnancy was defined as a transient increase in β-hCG levels, and clinical pregnancy was determined by the presence of a gestational sac in ultrasound five weeks post-IUI.
When the proposal was approved and the ethics code obtained, eligible patient files from the records unit at Shahid Beheshti Infertility Center were reviewed. The researcher extracted data from these files into the study forms and then entered them into SPSS 16 software. The parameters collected included maternal age, body mass index (BMI), duration of infertility, male factor infertility, female factor infertility (endometriosis, diminished ovarian reserve (DOR), advanced age, polycystic ovarian syndrome (PCOS)), endometrial thickness (<8 mm, ≥8 mm), the number of mature follicles, the number of IUI cycles, and the type of ovulation-stimulating medication used.
SPSS was used for data analysis. Descriptive statistics were employed for quantitative parameters, including central tendency and dispersion indices. Frequency distributions were used to describe qualitative parameters. The absolute and relative frequencies of biochemical pregnancy and clinical pregnancy following IUI treatment were presented. Pregnancy and clinical pregnancy frequencies were also presented by background variables such as maternal age, BMI, and duration of infertility using cross-tabulations for qualitative parameters and means with standard deviations for quantitative parameters. Appropriate statistical tests were used for comparisons, including chi-square and Fisher's exact test for cross-tabulations, and an independent t-test for comparing quantitative parameters between two groups (α≤0.05).
Findings
In this study, 334 patients with infertility were examined. The average age of the patients in the study was 30.44±4.97 years. The mean Body Mass Index (BMI) of the patients was 26.66±4.19kg/m². The average number of IUI cycles among the patients was 1.67±0.76, and the average number of follicles during the last IUI cycle was 2.50±1.39 (Table 1).
Table 1. Demographic and Clinical Characteristics of the Studied Patients

Patients underwent an average of 1.67±0.76 IUI cycles (with a minimum of 1 cycle and a maximum of 5 cycles). Additionally, they had an average of 2.50±1.39 follicles during the last IUI procedure (with a minimum of 1 follicle and a maximum of 7 follicles). Among the patients, 59.9% (n=201) had an endometrial thickness of less than 8 mm. Biochemical pregnancy occurred in 20.7% of the patients, while clinical pregnancy was observed in 13.8%.
The average age of patients with positive biochemical pregnancy was 28.99±4.66 years, compared to 30.82±4.99 years for those with negative results. The average BMI was 26.84±4.77kg/m² for those with positive biochemical pregnancy and 26.62±4.03kg/m² for those with negative results. The average number of IUI cycles was 1.58±0.69 for positive cases and 1.69±0.78 for negative cases. The average number of follicles was 2.67±1.45 for patients with positive results and 2.46±1.38 for those with negative results. Regarding the comparison of parameters based on pregnancy outcomes, the mean age of patients with positive biochemical pregnancy results was significantly lower than those with negative results (p<0.006). Similarly, the mean age of patients with positive clinical pregnancy outcomes was significantly lower than those with negative outcomes (p<0.008). Moreover, a significant relationship was observed between biochemical pregnancy rates and endometrial thickness (p<0.002), indicating that patients with thicker endometrium responded better to treatment and had higher pregnancy rates. A significant association between clinical pregnancy rates and endometrial thickness was also found (p<0.007). Overall, younger patients and those with greater endometrial thickness experienced higher rates of both biochemical and clinical pregnancy (Table 2).
Table 2. Comparison of parameters based on biochemical and clinical pregnancy outcomes

Discussion
The aim of this study was to identify factors associated with biochemical and clinical pregnancy rates after IUI treatment over a 5-year period. Among infertility treatments, IUI, with or without ovulation stimulation, is the first-line therapy as it is relatively inexpensive, non-invasive, and has a reasonably acceptable efficacy [9-11]. In this study, the biochemical pregnancy rate was 20.7%, and the clinical pregnancy rate was 13.8%. The biochemical pregnancy rate per cycle was 12.2%, and the clinical pregnancy rate per cycle was 8.1%. Similar to our study, Craig et al. in the U.S. have analyzed 1959 IUI cycles in 661 women, reporting a pregnancy rate per cycle of 15.3% [31]. Additionally, Ahmed et al. in Oman have studied 227 women undergoing ovulation stimulation with clomiphene citrate and letrozole, with or without gonadotropins, and report an overall pregnancy rate of 21.58% [32]. These studies show pregnancy rates comparable to our findings.
BMI is an influential factor for pregnancy success and the effectiveness of IUI in ovulation stimulation cycles. The average BMI was 26.66 kg/m². However, BMI was not found to significantly affect IUI success. In contrast, Craig et al. report higher biochemical pregnancy rates in overweight and morbidly obese individuals compared to those with a normal BMI, and higher clinical pregnancy rates in overweight individuals compared to other BMI groups [31]. Similarly, Dodson et al. find out lower pregnancy rates in underweight women compared to normal and overweight women, despite adjusting for age and ovulation stimulation medication dosage. Although obese women require more medication and have lower E2 levels compared to overweight and normal-weight women, no significant difference in pregnancy rates among the BMI groups is observed, similar to our findings [33].
Female age is a crucial factor in pregnancy success and IUI outcomes. As age increases, pregnancy rates decrease. Our study confirmed this, showing a significant decrease in both biochemical and clinical pregnancy rates with increasing age. In line with this, Wang et al. have observed a noticeable decline in clinical pregnancy rates with age, with higher rates in women under 25 compared to those aged 25-30 and over 35 [34]. Likewise, Merviel et al. have analyzed 1038 IUI cycles in 353 couples and identify female age as the strongest factor influencing pregnancy rates, while infertility type and smoking status, as in our study, are not significant factors [22]. No significant relationship was found between the husband's smoking status and the biochemical or clinical pregnancy rates.
Infertility causes can affect IUI success. However, no significant relationship between different infertility causes and biochemical or clinical pregnancy rates was detected. The highest biochemical pregnancy rates were observed in patients with PCOS, while the highest clinical pregnancy rates were associated with male-factor infertility. Dickey et al., in a study of 8051 IUI cycles involving 4000 couples, also find out no significant relationship between infertility causes and pregnancy rates [35,36]. Auj and Farhamand's study show a decline in pregnancy rates with increasing age, although this is not statistically significant, and pregnancy rates are similar across different infertility causes [37].
The type of ovulation stimulation medication can influence IUI success. In this study, the type of medication did not significantly affect biochemical pregnancy rates (p=0.237) or clinical pregnancy rates (p=0.363). However, excluding the group treated only with letrozole, those receiving HMG+FSH in addition to letrozole had higher pregnancy and clinical pregnancy rates. Similarly, Diamond et al. show that clinical pregnancy rates in IUI cycles after ovulation stimulation with letrozole are significantly lower than with gonadotropins [38].
The duration of infertility can impact IUI success. In our study, no significant relationship was found between the duration of infertility and biochemical or clinical pregnancy rates. However, Huniadi et al. show that longer infertility duration reduces IUI success, with 80% of pregnancies occurring in cases of infertility lasting less than four years [39-40].
Endometrial thickness is another important factor in IUI success. Our study found a significant relationship between endometrial thickness and both biochemical and clinical pregnancy rates, with pregnancy rates increasing as endometrial thickness increased. Consistent with our results, Jayakrishnan et al. have studied 3851 IUI cycles in couples with male-factor infertility, anovulation, endometriosis, or unexplained infertility. They reported that IUI success increases with endometrial thickness, with the highest pregnancy rate (41%) observed at a thickness of 9-10 mm [41].
The number of IUI cycles and mature follicles per cycle can also affect IUI success. In the current study, as the number of cycles increased, pregnancy and clinical pregnancy rates decreased, but no significant relationship was found between the number of cycles and pregnancy rates. Similarly, no significant relationship was found between the number of follicles and pregnancy rates. Ahmed et al. also report a decrease in IUI success with an increasing number of cycles and no significant relationship between follicle number and biochemical or clinical pregnancy rates [32].
It should be noted that this study had some limitations. The BMI of the male partners was not recorded, which could have influenced IUI success. Some required information was not available in the patient records and was obtained via telephone interviews. Additionally, the number of eligible participants was smaller than the calculated sample size, which limited the study.
Given that age is a key factor affecting infertility, there is a clear need for education and counseling regarding earlier marriages and avoiding prolonged contraception. It is recommended that midwives and fertility counselors discuss the impact of age and endometrial thickness on the prognosis of IUI treatment with patients and suggest alternative advanced treatments if the prognosis is poor.
Conclusion
As the woman's age increases, the rates of pregnancy and clinical pregnancy decrease. Additionally, endometrial thickness significantly influences pregnancy and clinical pregnancy rates, with increased thickness leading to higher pregnancy and clinical pregnancy rates.
Acknowledgments: The authors thank the staff of Kashn infertility clinic and shahid Beheshti hospital.
Ethical Permissions: The study was followed the Declaration of Helsinki Guideline and approved by the ethics committee of Kashan University of Medical Sciences Ethics Committee with ethical code IR.KUMS.MEDNT.REC.1401.232. Written consent was obtained from all participates.
Conflicts of Interests: The authors declared no conflict of interest in this manuscript.
Authors’ Contribution: Froozanfard F (First Author), Methodologist/Main Researcher (30%); Gilasi H (Second Author), Methodologist/Statistical Analyst (20%); Maboodi M S (Third Author), Introduction Writer/Assistant Researcher (20%); Taghavi S A (Fourth Author), Introduction Writer/Assistant Researcher/Discussion Writer (30%).
Funding/Support: This study was funded by a grant from Kashan University of Medical Sciences.
Keywords:
References
1. Vander Borght M, Wyns C. Fertility and infertility: Definition and epidemiology. Clin Biochem. 2018;62:2-10. [Link] [DOI:10.1016/j.clinbiochem.2018.03.012]
2. Zegers-Hochschild F, Adamson GD, De Mouzon J, Ishihara O, Mansour R, Nygren K, et al. The international committee for monitoring assisted reproductive technology (ICMART) and the World Health Organization (WHO) revised glossary on ART terminology, 2009. Hum Reprod. 2009;24(11):2683-7. [Link] [DOI:10.1093/humrep/dep343]
3. Taylor HS, Pal L, Sell E. Speroff's clinical gynecologic endocrinology and infertility. Philadelphia: Lippincott Williams & Wilkins; 2019. [Link]
4. Guan H, Tang H, Pan L, Song H, Tang L. Pregnancy predictors in unexplained infertility after intrauterine insemination. J Gynecol Obstet Hum Reprod. 2021;50(8):102071. [Link] [DOI:10.1016/j.jogoh.2021.102071]
5. Hammarberg K, Kirkman M. Infertility in resource-constrained settings: Moving towards amelioration. Reprod Biomed Online. 2013;26(2):189-95. [Link] [DOI:10.1016/j.rbmo.2012.11.009]
6. Luke B. Pregnancy and birth outcomes in couples with infertility with and without assisted reproductive technology: With an emphasis on US population-based studies. Am J Obstet Gynecol. 2017;217(3):270-81. [Link] [DOI:10.1016/j.ajog.2017.03.012]
7. Schlegel PN, Sigman M, Collura B, De Jonge CJ, Eisenberg ML, Lamb DJ, et al. Diagnosis and treatment of infertility in men: AUA/ASRM Guideline Part I. J Urol. 2021 ;205(1):36-43. [Link] [DOI:10.1097/JU.0000000000001521]
8. Soysal C, Ozmen U. Intrauterine insemination in ovulatory infertile patients. Niger J Clin Pract. 2018;21(10):1374-9. [Link] [DOI:10.4103/njcp.njcp_64_17]
9. Homburg R. IUI is a better alternative than IVF as the first-line treatment of unexplained infertility. Reprod BioMed Online. 2022;45(1):1-3. [Link] [DOI:10.1016/j.rbmo.2021.12.015]
10. Practice Committee of the American Society for Reproductive Medicine. Effectiveness and treatment for unexplained infertility. Fertil Steril. 2006;86(5 Suppl 1):S111-4. [Link] [DOI:10.1016/j.fertnstert.2006.07.1475]
11. Merviel P, Cabry R, Lourdel E, Barbier F, Scheffler F, Mansouri N, et al. Intrauterine insemination. La Revue du Praticien. 2014;64(1):87-91. [French] [Link]
12. Erdem A, Erdem M, Atmaca S, Korucuoglu U, Karabacak O. Factors affecting live birth rate in intrauterine insemination cycles with recombinant gonadotrophin stimulation. Reprod Biomed Online. 2008;17(2):199-206. [Link] [DOI:10.1016/S1472-6483(10)60195-2]
13. Turgay B, Şükür YE, Özmen B, Aytaç R, Atabekoğlu CS, Berker B, et al. Does different subfertility etiology affect pregnancy rates in intrauterine insemination cycles?. Turk J Med Sci. 2019;49(5):1439-43. [Link] [DOI:10.3906/sag-1902-200]
14. Starosta A, Gordon CE, Hornstein MD. Predictive factors for intrauterine insemination outcomes: A review. Fertil Res Pract. 2020;6(1):23. [Link] [DOI:10.1186/s40738-020-00092-1]
15. Osmanlıoğlu Ş, Şükür YE, Tokgöz VY, Özmen B, Sönmezer M, Berker B, et al. Intrauterine insemination with ovarian stimulation is a successful step prior to assisted reproductive technology for couples with unexplained infertility. J Obstet Gynaecol. 2022;42(3):472-7. [Link] [DOI:10.1080/01443615.2021.1916805]
16. Peeraer K, Debrock S, De Loecker P, Tomassetti C, Laenen A, Welkenhuysen M, et al. Low-dose human menopausal gonadotrophin versus clomiphene citrate in subfertile couples treated with intrauterine insemination: A randomized controlled trial. Hum Reprod. 2015;30(5):1079-88. [Link] [DOI:10.1093/humrep/dev062]
17. Cantineau AE, Rutten AG, Cohlen BJ. Agents for ovarian stimulation for intrauterine insemination (IUI) in ovulatory women with infertility. Cochrane Database Syst Rev. 2021;11(11). [Link] [DOI:10.1002/14651858.CD005356.pub3]
18. Ayeleke RO, Asseler JD, Cohlen BJ, Veltman-Verhulst SM. Intra‐uterine insemination for unexplained subfertility. Cochrane Database Syst Rev. 2020;3(3):CD001838. [Link] [DOI:10.1002/14651858.CD001838.pub6]
19. Van Eekelen R, Wang R, Danhof NA, Mol F, Mochtar M, Mol BW, et al. Cost-effectiveness of ovarian stimulation agents for IUI in couples with unexplained subfertility. Hum Reprod. 2021;36(5):1288-95. [Link] [DOI:10.1093/humrep/deab013]
20. Custers IM, Flierman PA, Maas P, Cox T, Van Dessel TJ, Gerards MH, et al. Immobilisation versus immediate mobilisation after intrauterine insemination: Randomised controlled trial. BMJ. 2009;339:b4080. [Link] [DOI:10.1136/bmj.b4080]
21. Gubert PG, Pudwell J, Van Vugt D, Reid RL, Velez MP. Number of motile spermatozoa inseminated and pregnancy outcomes in intrauterine insemination. Fertil Res Pract. 2019;5:10. [Link] [DOI:10.1186/s40738-019-0062-z]
22. Merviel P, Heraud MH, Grenier N, Lourdel E, Sanguinet P, Copin H. Predictive factors for pregnancy after intrauterine insemination (IUI): An analysis of 1038 cycles and a review of the literature. Fertil Steril. 2010;93(1):79-88. [Link] [DOI:10.1016/j.fertnstert.2008.09.058]
23. Huang S, Wang R, Yan H, Li N, Wang H, Luo L, et al. Intrauterine insemination (IUI) with or without letrozole for unexplained or mild male factor infertility: A randomized pilot study. Eur J Obstet Gynecol Reprod Biol. 2021;262:216-20. [Link] [DOI:10.1016/j.ejogrb.2021.05.029]
24. Jeon YE, Jung JA, Kim HY, Seo SK, Cho S, Choi YS, et al. Predictive factors for pregnancy during the first four intrauterine insemination cycles using gonadotropin. Gynecol Endocrinol. 2013;29(9):834-8. [Link] [DOI:10.3109/09513590.2013.808324]
25. . Nikbakht R, Saharkhiz N. The influence of sperm morphology, total motile sperm count of semen and the number of motile sperm inseminated in sperm samples on the success of intrauterine insemination. Int J Fertil Steril. 2011 Oct;5(3):168-73. [Link]
26. Mohammadi F, Mehdinia Z, Ghasemi S, Zolfaghari Z, Amjadi FS, Ashrafi M, et al. Relationship between sperm parameters and clinical outcomes of Intra Uterine Insemination (IUI). Caspian J Intern Med. 2021;12(1):70-6. [Link]
27. Ombelet W, Dhont N, Thijssen A, Bosmans E, Kruger T. Semen quality and prediction of IUI success in male subfertility: A systematic review. Reprod Biomed Online. 2014;28(3):300-9. [Link] [DOI:10.1016/j.rbmo.2013.10.023]
28. Kennedy S, Bergqvist A, Chapron C, D'Hooghe T, Dunselman G, Greb R, et al. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20(10):2698-704. [Link] [DOI:10.1093/humrep/dei135]
29. Goverde AJ, McDonnell J, Vermeiden JP, Schats R, Rutten FF, Schoemaker J. Intrauterine insemination or in-vitro fertilisation in idiopathic subfertility and male subfertility: a randomised trial and cost-effectiveness analysis. Lancet. 2000;355(9197):13-8. [Link] [DOI:10.1016/S0140-6736(99)04002-7]
30. Starosta A, Gordon CE, Hornstein MD. Predictive factors for intrauterine insemination outcomes: A review. Fertil Res Pract. 2020;6(1):23. [Link] [DOI:10.1186/s40738-020-00092-1]
31. Craig LB, Jarshaw CL, Hansen KR, Peck JD. Association between obesity and fecundity in patients undergoing intrauterine insemination. F S Rep. 2023;4(3):270-8. [Link] [DOI:10.1016/j.xfre.2023.05.003]
32. Ahmed B, Gowri V, Silja A, Alsabti J, Al-Khaduri M, Pathare A. Factors Influencing the succes Rate of intrauterine insemination: A retrospective study in Sultan Qaboos University Hospital. J Womens Health Care. 2017;6(5):1000402. [Link] [DOI:10.4172/2167-0420.1000402]
33. Dodson WC, Kunselman AR, Legro RS. Association of obesity with treatment outcomes in ovulatory infertile women undergoing superovulation and intrauterine insemination. Fertil Steril. 2006;86(3):642-6. [Link] [DOI:10.1016/j.fertnstert.2006.01.040]
34. Wang X, Zhang Y, Sun HL, Wang LT, Li XF, Wang F, et al. Factors affecting artificial insemination pregnancy outcome. Int J Gen Med. 2021;14:3961-9. [Link] [DOI:10.2147/IJGM.S312766]
35. Sahakyan M, Harlow BL, Hornstein MD. Influence of age, diagnosis, and cycle number on pregnancy rates with gonadotropin-induced controlled ovarian hyperstimulation and intrauterine insemination. Fertil Steril. 1999;72(3):500-4. [Link] [DOI:10.1016/S0015-0282(99)00300-3]
36. Dickey RP, Taylor SN, Lu PY, Sartor BM, Rye PH, Pyrzak R. Effect of diagnosis, age, sperm quality, and number of preovulatory follicles on the outcome of multiple cycles of clomiphene citrate-intrauterine insemination. Fertil Steril. 2002;78(5):1088-95. [Link] [DOI:10.1016/S0015-0282(02)04212-7]
37. Auj M, Farhamand F. Investigating the effect of age and the number of times IUI and the cause of infertility on the pregnancy rate in IUI and COH. Tehran: Academic Jihad Center; 2002. [Persian] [Link]
38. Diamond MP, Legro RS, Coutifaris C, Alvero R, Robinson RD, Casson P, et al. Letrozole, gonadotropin, or clomiphene for unexplained infertility. N Engl J Med. 2015;373(13):1230-40. [Link] [DOI:10.1056/NEJMoa1414827]
39. Legro RS, Brzyski RG, Diamond MP, Coutifaris C, Schlaff WD, Casson P, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371(2):119-29. [Link] [DOI:10.1056/NEJMoa1313517]
40. Huniadi A, Bimbo-Szuhai E, Botea M, Zaha I, Beiusanu C, Pallag A, et al. Fertility predictors in intrauterine insemination (IUI). J Pers Med. 2023;13(3):395. [Link] [DOI:10.3390/jpm13030395]
41. Jayakrishnan K, Sneha Ann A, Divya N. Factors affecting success of intrauterine insemination: A 3 year prospective study. Int J Reprod Contracept Obstet Gynecol. 2016;5(4):1077-83. [Link] [DOI:10.18203/2320-1770.ijrcog20160861]










