Volume 5, Issue 4 (2024)
J Clinic Care Skill 2024, 5(4): 233-239 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2024/09/22 | Accepted: 2024/11/21 | Published: 2024/11/30
Received: 2024/09/22 | Accepted: 2024/11/21 | Published: 2024/11/30
How to cite this article
Zareifrar N, Sharifi A, Khosravani S, Zoladl M, Khoramrooz S, Sharifi K. Comparison of the Antibacterial Activity of Hydroalcoholic Extracts of Cydonia oblonga and Hypericum perforatum with Clindamycin. J Clinic Care Skill 2024; 5 (4) :233-239
URL: http://jccs.yums.ac.ir/article-1-302-en.html
URL: http://jccs.yums.ac.ir/article-1-302-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
N. Zareifrar1 
 , A. Sharifi *2
, A. Sharifi *2 
 , S.A. Khosravani2
, S.A. Khosravani2 
 , M. Zoladl3
, M. Zoladl3 
 , S.S. Khoramrooz2
, S.S. Khoramrooz2 
 , K. Sharifi4
, K. Sharifi4 


 , A. Sharifi *2
, A. Sharifi *2 
 , S.A. Khosravani2
, S.A. Khosravani2 
 , M. Zoladl3
, M. Zoladl3 
 , S.S. Khoramrooz2
, S.S. Khoramrooz2 
 , K. Sharifi4
, K. Sharifi4 

1- Student Research Committee, Yasuj University of Medical Sciences, Yasuj, Iran
2- Cellular and Molecular Research Center, Yasuj University of Medical Sciences, Yasuj, Iran
3- Social Determinants of Health Research Center, Yasuj University of Medical Sciences, Yasuj, Iran
4- Student Research Committee, Kazerun Branch, Islamic Azad University, Kazerun, Iran
2- Cellular and Molecular Research Center, Yasuj University of Medical Sciences, Yasuj, Iran
3- Social Determinants of Health Research Center, Yasuj University of Medical Sciences, Yasuj, Iran
4- Student Research Committee, Kazerun Branch, Islamic Azad University, Kazerun, Iran
Full-Text (HTML) (736 Views)
Introduction
Hospital-acquired infection is an infection that people who are hospitalized contract during their stay in the hospital, and the symptoms of the disease may appear during hospitalization or after the patient is discharged [1, 2]. Infections that appear after 48 hours are usually considered hospital-acquired infections [1, 3]. The individual is likely to be in the latent stage of the disease when admitted to the hospital. Manifestations of the disease may occur during hospitalization or after the patient is discharged [2, 4]. The prevalence of hospital-acquired infections has been a major health and medical problem, and as the patient's length of stay in the hospital increases, the likelihood of infection and mortality from it increases, leading to increased medical costs [5]. According to the report of the World Health Organization, approximately 15% of hospitalized patients suffer from this infection. The incidence of hospital infections in Iran has been reported from at least 1.9% to more than 25%. The treatment of hospital infections is very difficult due to the resistance of most microbial strains and is very expensive due to the prolonged hospitalization time of patients. Bacteria alone cause about 90% of hospital infections [6-8]. Common bacteria causing hospital infection are Pseudomonas aeruginosa, Escherichia coli, Acinetobacter baumannii, Staphylococcus aureus, and Enterococci [9, 10].
Staphylococcus aureus (S. aureus) is a Gram-positive bacterium that is a major pathogen and cause of infectious diseases in humans and animals [11]. The rate of bacterial colonization in the skin and nasal mucosa is approximately 30% of the total. This is a common hospital-acquired opportunistic infection that affects all body tissues and causes bacteremia, infective endocarditis, soft tissue and skin infections, osteomyelitis, arthritis, pneumonia, meningitis, urinary tract infection, gastroenteritis, and toxic shock. If hospitalized with this infection, the risk of death increases [12]. Staphylococcus aureus secretes extracellular polymeric substances (EPS), called biofilms, which contribute to microbial resistance and reduce the effectiveness of antibiotics [11]. Biofilms are complex, tightly packed microbial populations surrounded by an extracellular polymeric matrix produced by the bacteria themselves. Biofilms account for 80% of infections in the body [13]. In Staphylococcus aureus, the biofilm contains 12 different genes including fibrinogen-binding proteins (fib) gene, fibronectin-binding proteins (fnbA and fnbB) genes, genes, clumping factor (clfA and B), elastin binding protein (ebps), intercellular adhesion (icaA, B, C and D), laminin binding protein (eno) and collagen binding protein (cna) gene [14]. However, when Staphylococcus aureus bacteria become resistant to any antibiotic, they can cause serious opportunistic infections or diseases. The U.S. Centers for Disease Control and Prevention (CDC) reports the prevalence of antibiotic resistance in staphylococci in the population at 5%. Staphylococcal resistance has been observed to antibiotics such as methicillin, cefazolin, penicillin, cloxacillin, oxacillin, cefoxitin, and other common antibiotics [15].
Clindamycin impedes ribosomal protein synthesis and reduces the production of multiple exotoxins. Clindamycin is the most widely used because of the lack of its inoculation effect, tendency to accumulate in abscesses, low cost, activity in the stationary phase, and the inhibitory activity of protein synthesis. Tolerability, and suppression of penicillin-inducible exotoxin production [16]. Clindamycin has a good effect on the skin and rapidly absorbed through the gastrointestinal tract and has a half-life similar to plasma of about 2 to 3 hours. After absorption, it is widely distributed in body tissues and fluids, especially bones, bile and urine. It is mainly excreted through urine. The use of the drug is limited. It is effective in the treatment of chronic bone and joint infections, chronic intrauterine infections, vaginal infections, Streptococcus pneumoniae bacteria, other streptococci, Staphylococcus aureus, and the anaerobic bacterium Bacteroides fragilis [17]. Studies have reported the prevalence of S. aureus resistance to clindamycin to be 7-34% worldwide. In a study in Nepal, 15 (39.5%) of clinical isolates of S. aureus showed methicillin resistance and 14 (36.5%) of clinical isolates showed inducible resistance to clindamycin [18].
Indiscriminate use of chemicals has led to the emergence of resistant microbial isolates, which increase in number every day. The emergence of strains resistant to chemical drugs makes it necessary to try to find new antimicrobial agents. Plants and their compounds, including essential oils and plant extracts, have the potential to replace chemical drugs. Plants and their compounds, including essential oils and plant extracts, have the potential to replace chemical drugs [19, 20]. This is despite the fact that the side effects of these compounds are less compared to chemical drugs. The use of medicinal plants for treatment has been at the same time as the history of human life. The tree (Cydonia oblonga Mill) is a small to medium-sized tree that reaches a height of 5 to 6 meters [21]. The scientific name is Cydonia oblonga Mill and in English it is called Quince and in Persian [22]. The active compounds of the Cydonia oblonga include flavonoids, phenolic acids, glycosides, tannins, sterols, pectin, lipids, vitamin C, alkaloids, resins, and amygdalin, which are considered to be effective and therapeutic ingredients of plants [23]. St. John's wort, also known by other names such as Hofariqon, tea grass, and with the scientific name Hypericum perforatum and the Latin name St. John's wort, is also called Goatweed, which is a valuable medicinal plant native to Western Europe, North Africa, and Asia [24, 25]. It contains various compounds and chemicals such as anthraquinone derivatives (naphthodianthrones), flavonoids, phloroglucinols, tannins, some phenols, volatile oils, hyperforin, and hypericin has antibacterial, antiviral, and anti-inflammatory activities [26, 27]. Clindamycin is an antibiotic from the lincosamide class [17].
The existence of complex and intelligent mechanisms that create resistance in bacteria has made the issue of bacterial resistance to antibiotics one of the problems of treatment systems and has revealed the need to discover and use antimicrobial drugs with greater effectiveness and less toxicity [23]. The prevalence of methicillin-resistant and inducible Staphylococcus aureus resistance to clindamycin depends on location and region. Data on the status of antibiotic resistance in a geographical area is essential for improving antibiotic use and providing guidance for treatment [18]. Moreover, despite the studies about the effect of the Cydonia oblonga, and Hypericum perforatum on S. aureus, their synergistic effects have not yet been studied. Therefore, the present study aimed to compare the antibacterial activity of hydroalcoholic extracts of Cydonia oblonga, Hypericum perforatum with Clindamycin and their synergistic effect against standard strain and clinical isolates of biofilm formation. Moreover, this study presents a genome of clinical isolates of biofilm formation of Staphylococcus aureus.
Materials and Methods
This experimental study was performed in the microbiology laboratory department of Medical Sciences Yasuj University, Yasuj, Iran in 2022.
Samples
Based on the results of previous studies [23, 28], ɑ=0.05, β=0.02, and below the formula, the sample size was calculated as 12.
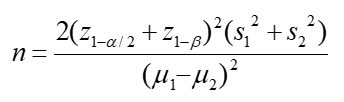
These samples were collected using simple random sampling. The samples of clinical isolates of S. aureus were taken from the noses of the staff of Imam Sajjad and Shahid Beheshti hospitals in Yasuj. Also, a standard strain sample (ATCC35556) of S. aureus was purchased from the National Research Center was incubated on the Mueller-Hinton Broth culture medium for 24 hours.
The collection of the plants
The Cydonia oblonga was collected during the fall season (from the end of September 2020) from Kohgiluyeh and Boyer-Ahmad Province, then the core was removed and dried in the dark at room temperature for 8 days. The Hypericum perforatum was collected in March 2021 from Dena Mountains, Kohgiluyeh and Boyer-Ahmad province, Iran. The collected parts were neatened and shade-dried.
Preparation of hydroalcoholic extract of the plants
For the preparation of hydroalcoholic extracts, 300 g of each dried plants were ground in a mill, and extraction was conducted by the maceration method. For this purpose, 70% alcohol was added to 300 g of each plant to a final volume of one liter; The resulting solution was stored for three days and then filtered through a Whatman filter paper. The filtrate was then concentrated in a rotary evaporator (Hyedolph, type: HeizbadHei-VAP, Germany) at 40°C. The extracts were stored at -20°C till later use [29].
Preparation of standard bacterial inoculum
In the present study, a standard strain sample (ATCC35556) of S. aureus was purchased from the National Research Center was incubated on the Mueller-Hinton Broth culture medium for 24 hours. Bacterial isolates were removed from storage, streaked onto Columbia agar plates supplemented with 5% sheep blood (bioMérieux) and incubated for 24 h at 37C in ambient air. A working bacterial suspension was prepared by suspending 4-5 isolated colonies in 5 mL of Mueller-Hinton broth. The turbidity of this suspension was carefully adjusted photometrically (630 nm) to equal that of a 0.5 McFarland standard.
Preparing microbial suspension
To prepare microbial suspension equal to 0.5 McFarland standard (105 CFU/ml), a 24-h culture was conducted on blood agar and then a suspension with 0.5 McFarland turbidity in normal saline was prepared. After conducting bacterial culture and preparing microbial suspension, broth microdilution in a sterile 96-well plate according to 0.5 McFarland standard (105 CFU/ml) was used to determine the antimicrobial effects of the extracts.
Determination of the Minimum Inhibitory Concentration (MIC)
To determine the MIC, the broth microdilution method was used according to CLSI standards. In this test, the concentrations of 390.63, 781.25, 1562.5, 3125, 6250, 12500 and 25000 μg/ml of the Cydonia oblonga extract and the concentrations of 46/88, 93.75, 187.5, 375, 750, 1500 and 3000 μg/ml of the Hypericum perforatum and the concentrations of 0.31, 0.63, 1.25, 2.5, 5, 10 and 20 μg/ml of the Clindamycin were tested. Finally, 10 μl of bacterial suspensions that were prepared were equal to Half McFarland tube (5×106 CFU/ml) added to all wells while the final volume per well was 100 μl. The first well was considered negative control (containing the culture medium and the extract) and the second well was considered to be positive control (containing the culture medium and the bacterium). Microplate was incubated for 24 hours in a shaking incubator at 37°C and the optical density (OD) of plates was read by an ELISA plate reader at a wavelength of 620 nm and the percentage of inhibition was measured. The test was conducted in triplicate [30].
Determination of the Minimum Bactericidal Concentration (MBC)
To determine the minimum bactericidal concentration (MBC), all wells without turbidity were separately cultured on the blood agar medium and incubated at 37°C for 24 hours. Then, by checking whether the colonization was done or not, the minimum concentrations of the extract in which the bacteria were not able to grow were considered to represent MBC [31].
Determination of zone of inhibition diameter (ZOI)
To determine the zone of inhibition, the Kirby Bauer method was used in bacterial suspension equal to Half McFarland (5×106 CFU/ml) was prepared and cultured on Mueller-Hinton agar medium (Pure plate method). 60 microliters of the MIC concentration of the extracts and antibiotic and the simultaneous use of the two extracts were poured into the wells. It was poured into each well-created. It should be noted that one well was also considered as a negative control, in which 60 microliters of DMSO was poured into it. Also, the antibiotic disk of clindamycin manufactured by Hi Media Company, India, was used as a positive control. Then, we incubated the plates at 37°C for 18-20 hours [32, 33].
Multiplex polymerase chain reaction (PCR)
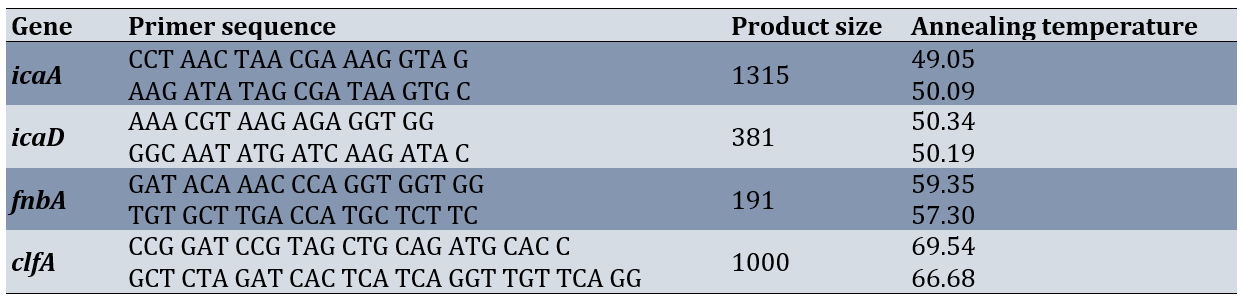
DNA extraction was performed by boiling method, several loops of bacteria (24 h) were boiled in a microtube containing sterile distilled water for 10 min at 100°C and then centrifuged. The supernatant was kept as template DNA for PCR. Each PCR reaction mixture (25 μl) contained 3 μl DNA template, 12.5 μl Mastermix (Pishgam, Iran), and 6 pmol of each primer and 7.5 μl distilled water. PCR amplifications were carried out in a thermal cycler.
The supernatant which was considered as a DNA template for qnr genes (icaA, icaD, fnbA and clfA) was detected via PCR using specific protocols and primers (Table 1) [8]. After amplification, 10 µL of the PCR products were electrophoresed (Major Science MP300, Taiwan) on 1.5% agarose gel (Pishgam, Iran) at 90 V for 45 minutes. The PCR products were stained with Gel Stain (Pishgam, Iran). They were then visualized by Gel Documentation (Major Science, Taiwan) [34].
Table 1. Specific primers and annealing temperature for genes amplification genes biofilm formation with part size
Statistical analysis
The data were analyzed by SPSS software version 16 using descriptive including mean (standard deviation) and inferential statistics such as one-way analysis of variance and Bonferroni post hoc test. p<0.05 was considered as a significant level.
Findings
From 148 clinical isolates of Staphylococcus aureus, 95 isolates (64.2%) of Staphylococcus aureus forming biofilm were isolated by Congord Agar phenotypic method which 12 isolates had genes during PCR.icaA.icaD clfA and fnbA. However, the relative frequency of simultaneous presence of fnbA, icaA, icaD, and clfA genes in polymerase chain reaction (PCR) in Staphylococcus aureus isolates that were isolated from nasal swabs of the participants, was 9.1%, and in biofilm-forming Staphylococcus aureus isolates was 12.63%.
The results indicated a significant difference in MIC between groups (p=0.001). Bonferroni's post hoc test showed these differences between the hydroalcoholic extract of the Cydonia oblonga with the Hypericum perforatum (P=0.031), and clindamycin (0.001), between Cydonia oblonga and Hypericum perforatum (Cydonia oblonga change) with each of groups (0.001; Table 2). Therefore, in terms of MIC, Clindamycin, Hypericum perforatum, Cydonia oblonga and Hypericum perforatum (concentration change of Hypericum perforatum), and Cydonia oblonga and Hypericum perforatum (concentration change Cydonia oblonga) were ranked, respectively, from lowest to highest (Table 2).
Table 2. Comparing MIC and MBC against clinical isolates of Staphylococcus aureus biofilm formation between groups
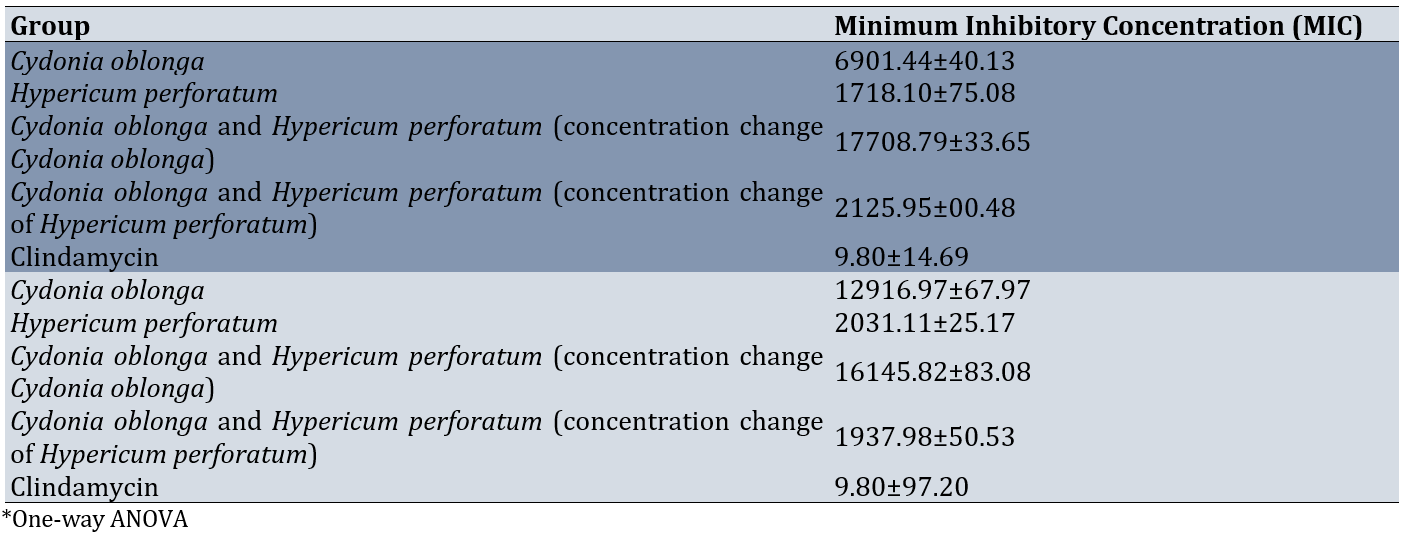
The results revealed significant differences in MBC between groups (p<0.001). Bonferroni's post hoc test showed these differences between hydroalcoholic extract of the Cydonia oblonga with the Hypericum perforatum (0.0001), the combined use of the hydroalcoholic extracts of the Cydonia oblonga and the Hypericum perforatum (concentration change of Hypericum perforatum; 0.0001), and clindamycin (0.0001), between Cydonia oblonga and Hypericum perforatum (Cydonia oblonga change) with each of the groups (0.0001) except to Cydonia oblonga. Therefore, in terms of MBC, Clindamycin, Cydonia oblonga and Hypericum perforatum (concentration change of Hypericum perforatum), Hypericum perforatum, Cydonia oblonga, and combination of Cydonia oblonga and Hypericum perforatum (concentration change Cydonia oblonga) were ranked, respectively, from lowest to highest (Table 2).
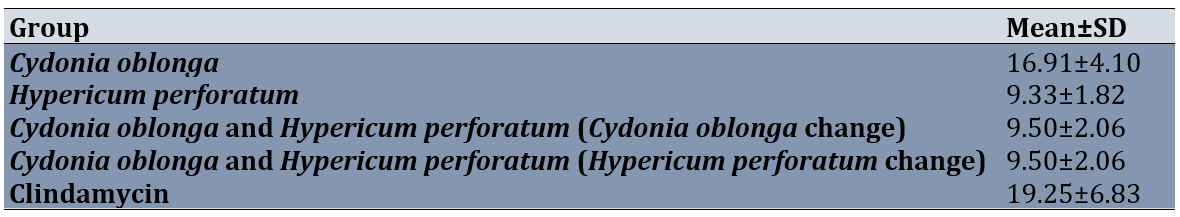
The results showed a significant difference in ZOI scores between groups (p=0.0001). However, Bonferroni's post hoc test showed these differences between the hydroalcoholic extract of the Cydonia oblonga with the Hypericum perforatum (0.0001), the combined use of the hydroalcoholic extracts of the Cydonia oblonga and the Hypericum perforatum (concentration change of Hypericum perforatum; 0.0001), and Clindamycin with each of groups (0.0001) except to Cydonia oblonga (Table 3).
Table 3. Comparing determination of zone of inhibition diameter (ZOI) against clinical isolates of Staphylococcus aureus biofilm formation between groups
Discussion
The present study aimed to compare the antibacterial activity of hydroalcoholic extracts of Cydonia oblonga, Hypericum perforatum with Clindamycin and their synergistic effect against standard strain and clinical isolates of biofilm formation. Moreover, this study presents a genome of clinical isolates of biofilm formation of Staphylococcus aureus.
Out of 148 clinical isolates of Staphylococcus aureus, 95 isolates of Staphylococcus aureus forming biofilm, with a relative frequency of 64.2%, of which 12 isolates had the clfA, fnbA, icaA, and icaD genes. Moreover, the relative frequency of the simultaneous presence of the clfA, fnbA, icaA, and icaD genes in the polymerase chain reaction was 19.19% and in the isolates of Staphylococcus aureus forming biofilms was 12.63%. This is consistent with the results of the study by Yazdani et al. in 2006 in Isfahan, where the frequency of biofilm production was 54% [35]. An important factor in increasing pathogenicity and resistance to antimicrobial agents is the ability to form biofilms [13]. Biofilm formation is a key mechanism for inhibiting the activity of antibiotics used against staphylococcal infections [36]. According to Wang et al., the frequency of biofilm production in Staphylococcus aureus isolates isolated from sputum samples of patients with lower respiratory tract infections producing biofilms is 70.6% [37]. Moreover, in the study by Coutinho et al., the gene frequency is 50% [38], and in the study by Mollaei et al., examining the frequency of genes encoding adhesion proteins in Staphylococcus aureus isolated from patients hospitalized in teaching hospitals in Zabol city, 50% of the isolates show the presence of at least one of the genes in question [39]. In the study by Demir et al., biofilm formation is observed in 79 (70.5%) of 112 Staphylococcus aureus isolates. In addition, 97 (86.6%) of all isolates are positive for icaA and icaD and 15 (13.4%) for bap. The adhesion genes, cna, fnbA, and clfA are identified in 98 (87.5%), 87 (77.7%), and 75 (66.9%) of the isolates, respectively [34].
The results of the present study indicated Clindamycin, Hypericum perforatum, and a combination of Cydonia oblonga and Hypericum perforatum (concentration change of Hypericum perforatum), had the lowest MIC, respectively than other groups. Moreover, the results of MBC revealed that clindamycin, a combination of Cydonia oblonga and Hypericum perforatum (concentration change in Hypericum perforatum), and Hypericum perforatum had lower MBC scores respectively than other groups. There was no significant difference between them.
In a study by Al-Khazraji, the antibacterial activity of ethanolic extract of Cydonia oblonga seeds is shown against Staphylococcus aureus. The chemical constituents of this plant include tannins, glycosides and flavonoids. The study also shows that the antibacterial activity of Cydonia oblonga seeds on Gram-positive bacteria is more than on Gram-negative bacteria [23]. However, in the present study, Cydonia oblonga seeds extract had the highest MIC and MBC against S. aureus forming biofilm compared to other interventions except for Cydonia oblonga and Hypericum perforatum (concentration change Cydonia oblonga). However, Hypericum perforatum had lower MIC and MBC compared to other interventions. Similar to the present study, Aygül & Şerbetçi indicate the antibacterial effects of Hypericum perforatum against S. aureus and inhibited the growth of biofilm formation and hemolytic activity, and the results show that the ethanolic extract of Hypericum perforatum inhibits biofilm formation and hemolytic activity [40].
Bahmani et al. in their study indicate the synergistic effects of hydroalcoholic extract of medicinal plants including Origanum vulgare and Hypericum perforatum against S. aureus [41]. In the present study, although the MBC of a combination of Cydonia oblonga and Hypericum perforatum with Concentration change in Hypericum perforatum is lower than in other interventions, however, there was no significant difference between the Hypericum perforatum group and the combination of Cydonia oblonga and Hypericum perforatum (concentration change in Hypericum perforatum).
Moreover, the results revealed that clindamycin, a combination of Cydonia oblonga and Hypericum perforatum (concentration change in Hypericum perforatum), and Hypericum perforatum respectively had higher ZOI scores than other groups. However, Clindamycin forms more ZOI, and Hypericum perforatum group, the combination of Cydonia oblonga and Hypericum perforatum (concentration change in Hypericum perforatum), and Cydonia oblonga and Hypericum perforatum (Cydonia oblonga change) were the same in ZOI. Of course, MIC and MBC are better indicators for judging the effect of the intervention against S. aureus biofilm.
One limitation of this study was that we only evaluated the Cydonia oblonga and Hypericum perforatum harvested at a specific time from a specific location. Also, microbial samples taken from the nose were used and microorganisms collected and purified from the oral cavity were not assessed. Thus, the results were limited to nose samples. In addition, another limitation was the low availability of strains pure bacterial.
It is better to examine toxicity tests on both cell cultures and laboratory animals before using them to ensure the safety of this plant on human cells. Therefore, a similar study in humans is suggested.
Conclusion
Clindamycin has the lowest MIC and MBC compared to other interventions. However, the extract of Hypericum perforatum has the lowest MIC against the S. aureus biofilm. Although the combination of the hydrochloric extract of the combination of Cydonia oblonga and Hypericum perforatum (concentration change of Hypericum perforatum) has the lowest bactericidal concentration, no significant difference is observed between the MBC of the Hypericum perforatum and the combination of the extract of the combination of Cydonia oblonga and Hypericum perforatum (concentration change of Hypericum perforatum).
Acknowledgments: This article is part of a master's thesis for a student. We would like to thank all those who supported this research.
Ethical Permissions: This study has been approved by the Research Ethics Committee of Yasuj University of Medical Sciences with code IR.YUMS.REC.1399.076.
Conflicts of Interests: This study has been supported by the Deputy of Research and Technology, Yasuj University of Medical Sciences.
Authors' Contribution: Zareifrar N (First Author), Main Researcher/Introduction Writer/Discussion Writer (15%); Sharifi A (Second Author), Main Researcher/Introduction Writer/Discussion Writer/Methodologist (25%); Khosravani SA (Third Author), Methodologist/Assistant Researcher/Statistical Analyst (15%); Zoladl M (Fourth Author), Assistant Researcher/Statistical Analyst/Discussion Writer (20%); Khoramrooz SS (Fifth Author), Methodologist/Assistant Researcher/Statistical Analyst (15%); Sharifi K (Sixth Author), Assistant Researcher/Introduction Writer (10%)
Funding/Support: This article is a part of Mis. Niloofar Zareifrar MSc thesis. This study has been supported by Yasuj University of Medical Sciences, Deputy of Research and Technology.
Hospital-acquired infection is an infection that people who are hospitalized contract during their stay in the hospital, and the symptoms of the disease may appear during hospitalization or after the patient is discharged [1, 2]. Infections that appear after 48 hours are usually considered hospital-acquired infections [1, 3]. The individual is likely to be in the latent stage of the disease when admitted to the hospital. Manifestations of the disease may occur during hospitalization or after the patient is discharged [2, 4]. The prevalence of hospital-acquired infections has been a major health and medical problem, and as the patient's length of stay in the hospital increases, the likelihood of infection and mortality from it increases, leading to increased medical costs [5]. According to the report of the World Health Organization, approximately 15% of hospitalized patients suffer from this infection. The incidence of hospital infections in Iran has been reported from at least 1.9% to more than 25%. The treatment of hospital infections is very difficult due to the resistance of most microbial strains and is very expensive due to the prolonged hospitalization time of patients. Bacteria alone cause about 90% of hospital infections [6-8]. Common bacteria causing hospital infection are Pseudomonas aeruginosa, Escherichia coli, Acinetobacter baumannii, Staphylococcus aureus, and Enterococci [9, 10].
Staphylococcus aureus (S. aureus) is a Gram-positive bacterium that is a major pathogen and cause of infectious diseases in humans and animals [11]. The rate of bacterial colonization in the skin and nasal mucosa is approximately 30% of the total. This is a common hospital-acquired opportunistic infection that affects all body tissues and causes bacteremia, infective endocarditis, soft tissue and skin infections, osteomyelitis, arthritis, pneumonia, meningitis, urinary tract infection, gastroenteritis, and toxic shock. If hospitalized with this infection, the risk of death increases [12]. Staphylococcus aureus secretes extracellular polymeric substances (EPS), called biofilms, which contribute to microbial resistance and reduce the effectiveness of antibiotics [11]. Biofilms are complex, tightly packed microbial populations surrounded by an extracellular polymeric matrix produced by the bacteria themselves. Biofilms account for 80% of infections in the body [13]. In Staphylococcus aureus, the biofilm contains 12 different genes including fibrinogen-binding proteins (fib) gene, fibronectin-binding proteins (fnbA and fnbB) genes, genes, clumping factor (clfA and B), elastin binding protein (ebps), intercellular adhesion (icaA, B, C and D), laminin binding protein (eno) and collagen binding protein (cna) gene [14]. However, when Staphylococcus aureus bacteria become resistant to any antibiotic, they can cause serious opportunistic infections or diseases. The U.S. Centers for Disease Control and Prevention (CDC) reports the prevalence of antibiotic resistance in staphylococci in the population at 5%. Staphylococcal resistance has been observed to antibiotics such as methicillin, cefazolin, penicillin, cloxacillin, oxacillin, cefoxitin, and other common antibiotics [15].
Clindamycin impedes ribosomal protein synthesis and reduces the production of multiple exotoxins. Clindamycin is the most widely used because of the lack of its inoculation effect, tendency to accumulate in abscesses, low cost, activity in the stationary phase, and the inhibitory activity of protein synthesis. Tolerability, and suppression of penicillin-inducible exotoxin production [16]. Clindamycin has a good effect on the skin and rapidly absorbed through the gastrointestinal tract and has a half-life similar to plasma of about 2 to 3 hours. After absorption, it is widely distributed in body tissues and fluids, especially bones, bile and urine. It is mainly excreted through urine. The use of the drug is limited. It is effective in the treatment of chronic bone and joint infections, chronic intrauterine infections, vaginal infections, Streptococcus pneumoniae bacteria, other streptococci, Staphylococcus aureus, and the anaerobic bacterium Bacteroides fragilis [17]. Studies have reported the prevalence of S. aureus resistance to clindamycin to be 7-34% worldwide. In a study in Nepal, 15 (39.5%) of clinical isolates of S. aureus showed methicillin resistance and 14 (36.5%) of clinical isolates showed inducible resistance to clindamycin [18].
Indiscriminate use of chemicals has led to the emergence of resistant microbial isolates, which increase in number every day. The emergence of strains resistant to chemical drugs makes it necessary to try to find new antimicrobial agents. Plants and their compounds, including essential oils and plant extracts, have the potential to replace chemical drugs. Plants and their compounds, including essential oils and plant extracts, have the potential to replace chemical drugs [19, 20]. This is despite the fact that the side effects of these compounds are less compared to chemical drugs. The use of medicinal plants for treatment has been at the same time as the history of human life. The tree (Cydonia oblonga Mill) is a small to medium-sized tree that reaches a height of 5 to 6 meters [21]. The scientific name is Cydonia oblonga Mill and in English it is called Quince and in Persian [22]. The active compounds of the Cydonia oblonga include flavonoids, phenolic acids, glycosides, tannins, sterols, pectin, lipids, vitamin C, alkaloids, resins, and amygdalin, which are considered to be effective and therapeutic ingredients of plants [23]. St. John's wort, also known by other names such as Hofariqon, tea grass, and with the scientific name Hypericum perforatum and the Latin name St. John's wort, is also called Goatweed, which is a valuable medicinal plant native to Western Europe, North Africa, and Asia [24, 25]. It contains various compounds and chemicals such as anthraquinone derivatives (naphthodianthrones), flavonoids, phloroglucinols, tannins, some phenols, volatile oils, hyperforin, and hypericin has antibacterial, antiviral, and anti-inflammatory activities [26, 27]. Clindamycin is an antibiotic from the lincosamide class [17].
The existence of complex and intelligent mechanisms that create resistance in bacteria has made the issue of bacterial resistance to antibiotics one of the problems of treatment systems and has revealed the need to discover and use antimicrobial drugs with greater effectiveness and less toxicity [23]. The prevalence of methicillin-resistant and inducible Staphylococcus aureus resistance to clindamycin depends on location and region. Data on the status of antibiotic resistance in a geographical area is essential for improving antibiotic use and providing guidance for treatment [18]. Moreover, despite the studies about the effect of the Cydonia oblonga, and Hypericum perforatum on S. aureus, their synergistic effects have not yet been studied. Therefore, the present study aimed to compare the antibacterial activity of hydroalcoholic extracts of Cydonia oblonga, Hypericum perforatum with Clindamycin and their synergistic effect against standard strain and clinical isolates of biofilm formation. Moreover, this study presents a genome of clinical isolates of biofilm formation of Staphylococcus aureus.
Materials and Methods
This experimental study was performed in the microbiology laboratory department of Medical Sciences Yasuj University, Yasuj, Iran in 2022.
Samples
Based on the results of previous studies [23, 28], ɑ=0.05, β=0.02, and below the formula, the sample size was calculated as 12.
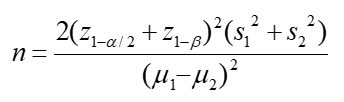
These samples were collected using simple random sampling. The samples of clinical isolates of S. aureus were taken from the noses of the staff of Imam Sajjad and Shahid Beheshti hospitals in Yasuj. Also, a standard strain sample (ATCC35556) of S. aureus was purchased from the National Research Center was incubated on the Mueller-Hinton Broth culture medium for 24 hours.
The collection of the plants
The Cydonia oblonga was collected during the fall season (from the end of September 2020) from Kohgiluyeh and Boyer-Ahmad Province, then the core was removed and dried in the dark at room temperature for 8 days. The Hypericum perforatum was collected in March 2021 from Dena Mountains, Kohgiluyeh and Boyer-Ahmad province, Iran. The collected parts were neatened and shade-dried.
Preparation of hydroalcoholic extract of the plants
For the preparation of hydroalcoholic extracts, 300 g of each dried plants were ground in a mill, and extraction was conducted by the maceration method. For this purpose, 70% alcohol was added to 300 g of each plant to a final volume of one liter; The resulting solution was stored for three days and then filtered through a Whatman filter paper. The filtrate was then concentrated in a rotary evaporator (Hyedolph, type: HeizbadHei-VAP, Germany) at 40°C. The extracts were stored at -20°C till later use [29].
Preparation of standard bacterial inoculum
In the present study, a standard strain sample (ATCC35556) of S. aureus was purchased from the National Research Center was incubated on the Mueller-Hinton Broth culture medium for 24 hours. Bacterial isolates were removed from storage, streaked onto Columbia agar plates supplemented with 5% sheep blood (bioMérieux) and incubated for 24 h at 37C in ambient air. A working bacterial suspension was prepared by suspending 4-5 isolated colonies in 5 mL of Mueller-Hinton broth. The turbidity of this suspension was carefully adjusted photometrically (630 nm) to equal that of a 0.5 McFarland standard.
Preparing microbial suspension
To prepare microbial suspension equal to 0.5 McFarland standard (105 CFU/ml), a 24-h culture was conducted on blood agar and then a suspension with 0.5 McFarland turbidity in normal saline was prepared. After conducting bacterial culture and preparing microbial suspension, broth microdilution in a sterile 96-well plate according to 0.5 McFarland standard (105 CFU/ml) was used to determine the antimicrobial effects of the extracts.
Determination of the Minimum Inhibitory Concentration (MIC)
To determine the MIC, the broth microdilution method was used according to CLSI standards. In this test, the concentrations of 390.63, 781.25, 1562.5, 3125, 6250, 12500 and 25000 μg/ml of the Cydonia oblonga extract and the concentrations of 46/88, 93.75, 187.5, 375, 750, 1500 and 3000 μg/ml of the Hypericum perforatum and the concentrations of 0.31, 0.63, 1.25, 2.5, 5, 10 and 20 μg/ml of the Clindamycin were tested. Finally, 10 μl of bacterial suspensions that were prepared were equal to Half McFarland tube (5×106 CFU/ml) added to all wells while the final volume per well was 100 μl. The first well was considered negative control (containing the culture medium and the extract) and the second well was considered to be positive control (containing the culture medium and the bacterium). Microplate was incubated for 24 hours in a shaking incubator at 37°C and the optical density (OD) of plates was read by an ELISA plate reader at a wavelength of 620 nm and the percentage of inhibition was measured. The test was conducted in triplicate [30].
Determination of the Minimum Bactericidal Concentration (MBC)
To determine the minimum bactericidal concentration (MBC), all wells without turbidity were separately cultured on the blood agar medium and incubated at 37°C for 24 hours. Then, by checking whether the colonization was done or not, the minimum concentrations of the extract in which the bacteria were not able to grow were considered to represent MBC [31].
Determination of zone of inhibition diameter (ZOI)
To determine the zone of inhibition, the Kirby Bauer method was used in bacterial suspension equal to Half McFarland (5×106 CFU/ml) was prepared and cultured on Mueller-Hinton agar medium (Pure plate method). 60 microliters of the MIC concentration of the extracts and antibiotic and the simultaneous use of the two extracts were poured into the wells. It was poured into each well-created. It should be noted that one well was also considered as a negative control, in which 60 microliters of DMSO was poured into it. Also, the antibiotic disk of clindamycin manufactured by Hi Media Company, India, was used as a positive control. Then, we incubated the plates at 37°C for 18-20 hours [32, 33].
Multiplex polymerase chain reaction (PCR)
DNA extraction was performed by boiling method, several loops of bacteria (24 h) were boiled in a microtube containing sterile distilled water for 10 min at 100°C and then centrifuged. The supernatant was kept as template DNA for PCR. Each PCR reaction mixture (25 μl) contained 3 μl DNA template, 12.5 μl Mastermix (Pishgam, Iran), and 6 pmol of each primer and 7.5 μl distilled water. PCR amplifications were carried out in a thermal cycler.
The supernatant which was considered as a DNA template for qnr genes (icaA, icaD, fnbA and clfA) was detected via PCR using specific protocols and primers (Table 1) [8]. After amplification, 10 µL of the PCR products were electrophoresed (Major Science MP300, Taiwan) on 1.5% agarose gel (Pishgam, Iran) at 90 V for 45 minutes. The PCR products were stained with Gel Stain (Pishgam, Iran). They were then visualized by Gel Documentation (Major Science, Taiwan) [34].
Table 1. Specific primers and annealing temperature for genes amplification genes biofilm formation with part size

Statistical analysis
The data were analyzed by SPSS software version 16 using descriptive including mean (standard deviation) and inferential statistics such as one-way analysis of variance and Bonferroni post hoc test. p<0.05 was considered as a significant level.
Findings
From 148 clinical isolates of Staphylococcus aureus, 95 isolates (64.2%) of Staphylococcus aureus forming biofilm were isolated by Congord Agar phenotypic method which 12 isolates had genes during PCR.icaA.icaD clfA and fnbA. However, the relative frequency of simultaneous presence of fnbA, icaA, icaD, and clfA genes in polymerase chain reaction (PCR) in Staphylococcus aureus isolates that were isolated from nasal swabs of the participants, was 9.1%, and in biofilm-forming Staphylococcus aureus isolates was 12.63%.
The results indicated a significant difference in MIC between groups (p=0.001). Bonferroni's post hoc test showed these differences between the hydroalcoholic extract of the Cydonia oblonga with the Hypericum perforatum (P=0.031), and clindamycin (0.001), between Cydonia oblonga and Hypericum perforatum (Cydonia oblonga change) with each of groups (0.001; Table 2). Therefore, in terms of MIC, Clindamycin, Hypericum perforatum, Cydonia oblonga and Hypericum perforatum (concentration change of Hypericum perforatum), and Cydonia oblonga and Hypericum perforatum (concentration change Cydonia oblonga) were ranked, respectively, from lowest to highest (Table 2).
Table 2. Comparing MIC and MBC against clinical isolates of Staphylococcus aureus biofilm formation between groups

The results revealed significant differences in MBC between groups (p<0.001). Bonferroni's post hoc test showed these differences between hydroalcoholic extract of the Cydonia oblonga with the Hypericum perforatum (0.0001), the combined use of the hydroalcoholic extracts of the Cydonia oblonga and the Hypericum perforatum (concentration change of Hypericum perforatum; 0.0001), and clindamycin (0.0001), between Cydonia oblonga and Hypericum perforatum (Cydonia oblonga change) with each of the groups (0.0001) except to Cydonia oblonga. Therefore, in terms of MBC, Clindamycin, Cydonia oblonga and Hypericum perforatum (concentration change of Hypericum perforatum), Hypericum perforatum, Cydonia oblonga, and combination of Cydonia oblonga and Hypericum perforatum (concentration change Cydonia oblonga) were ranked, respectively, from lowest to highest (Table 2).
The results showed a significant difference in ZOI scores between groups (p=0.0001). However, Bonferroni's post hoc test showed these differences between the hydroalcoholic extract of the Cydonia oblonga with the Hypericum perforatum (0.0001), the combined use of the hydroalcoholic extracts of the Cydonia oblonga and the Hypericum perforatum (concentration change of Hypericum perforatum; 0.0001), and Clindamycin with each of groups (0.0001) except to Cydonia oblonga (Table 3).
Table 3. Comparing determination of zone of inhibition diameter (ZOI) against clinical isolates of Staphylococcus aureus biofilm formation between groups

Discussion
The present study aimed to compare the antibacterial activity of hydroalcoholic extracts of Cydonia oblonga, Hypericum perforatum with Clindamycin and their synergistic effect against standard strain and clinical isolates of biofilm formation. Moreover, this study presents a genome of clinical isolates of biofilm formation of Staphylococcus aureus.
Out of 148 clinical isolates of Staphylococcus aureus, 95 isolates of Staphylococcus aureus forming biofilm, with a relative frequency of 64.2%, of which 12 isolates had the clfA, fnbA, icaA, and icaD genes. Moreover, the relative frequency of the simultaneous presence of the clfA, fnbA, icaA, and icaD genes in the polymerase chain reaction was 19.19% and in the isolates of Staphylococcus aureus forming biofilms was 12.63%. This is consistent with the results of the study by Yazdani et al. in 2006 in Isfahan, where the frequency of biofilm production was 54% [35]. An important factor in increasing pathogenicity and resistance to antimicrobial agents is the ability to form biofilms [13]. Biofilm formation is a key mechanism for inhibiting the activity of antibiotics used against staphylococcal infections [36]. According to Wang et al., the frequency of biofilm production in Staphylococcus aureus isolates isolated from sputum samples of patients with lower respiratory tract infections producing biofilms is 70.6% [37]. Moreover, in the study by Coutinho et al., the gene frequency is 50% [38], and in the study by Mollaei et al., examining the frequency of genes encoding adhesion proteins in Staphylococcus aureus isolated from patients hospitalized in teaching hospitals in Zabol city, 50% of the isolates show the presence of at least one of the genes in question [39]. In the study by Demir et al., biofilm formation is observed in 79 (70.5%) of 112 Staphylococcus aureus isolates. In addition, 97 (86.6%) of all isolates are positive for icaA and icaD and 15 (13.4%) for bap. The adhesion genes, cna, fnbA, and clfA are identified in 98 (87.5%), 87 (77.7%), and 75 (66.9%) of the isolates, respectively [34].
The results of the present study indicated Clindamycin, Hypericum perforatum, and a combination of Cydonia oblonga and Hypericum perforatum (concentration change of Hypericum perforatum), had the lowest MIC, respectively than other groups. Moreover, the results of MBC revealed that clindamycin, a combination of Cydonia oblonga and Hypericum perforatum (concentration change in Hypericum perforatum), and Hypericum perforatum had lower MBC scores respectively than other groups. There was no significant difference between them.
In a study by Al-Khazraji, the antibacterial activity of ethanolic extract of Cydonia oblonga seeds is shown against Staphylococcus aureus. The chemical constituents of this plant include tannins, glycosides and flavonoids. The study also shows that the antibacterial activity of Cydonia oblonga seeds on Gram-positive bacteria is more than on Gram-negative bacteria [23]. However, in the present study, Cydonia oblonga seeds extract had the highest MIC and MBC against S. aureus forming biofilm compared to other interventions except for Cydonia oblonga and Hypericum perforatum (concentration change Cydonia oblonga). However, Hypericum perforatum had lower MIC and MBC compared to other interventions. Similar to the present study, Aygül & Şerbetçi indicate the antibacterial effects of Hypericum perforatum against S. aureus and inhibited the growth of biofilm formation and hemolytic activity, and the results show that the ethanolic extract of Hypericum perforatum inhibits biofilm formation and hemolytic activity [40].
Bahmani et al. in their study indicate the synergistic effects of hydroalcoholic extract of medicinal plants including Origanum vulgare and Hypericum perforatum against S. aureus [41]. In the present study, although the MBC of a combination of Cydonia oblonga and Hypericum perforatum with Concentration change in Hypericum perforatum is lower than in other interventions, however, there was no significant difference between the Hypericum perforatum group and the combination of Cydonia oblonga and Hypericum perforatum (concentration change in Hypericum perforatum).
Moreover, the results revealed that clindamycin, a combination of Cydonia oblonga and Hypericum perforatum (concentration change in Hypericum perforatum), and Hypericum perforatum respectively had higher ZOI scores than other groups. However, Clindamycin forms more ZOI, and Hypericum perforatum group, the combination of Cydonia oblonga and Hypericum perforatum (concentration change in Hypericum perforatum), and Cydonia oblonga and Hypericum perforatum (Cydonia oblonga change) were the same in ZOI. Of course, MIC and MBC are better indicators for judging the effect of the intervention against S. aureus biofilm.
One limitation of this study was that we only evaluated the Cydonia oblonga and Hypericum perforatum harvested at a specific time from a specific location. Also, microbial samples taken from the nose were used and microorganisms collected and purified from the oral cavity were not assessed. Thus, the results were limited to nose samples. In addition, another limitation was the low availability of strains pure bacterial.
It is better to examine toxicity tests on both cell cultures and laboratory animals before using them to ensure the safety of this plant on human cells. Therefore, a similar study in humans is suggested.
Conclusion
Clindamycin has the lowest MIC and MBC compared to other interventions. However, the extract of Hypericum perforatum has the lowest MIC against the S. aureus biofilm. Although the combination of the hydrochloric extract of the combination of Cydonia oblonga and Hypericum perforatum (concentration change of Hypericum perforatum) has the lowest bactericidal concentration, no significant difference is observed between the MBC of the Hypericum perforatum and the combination of the extract of the combination of Cydonia oblonga and Hypericum perforatum (concentration change of Hypericum perforatum).
Acknowledgments: This article is part of a master's thesis for a student. We would like to thank all those who supported this research.
Ethical Permissions: This study has been approved by the Research Ethics Committee of Yasuj University of Medical Sciences with code IR.YUMS.REC.1399.076.
Conflicts of Interests: This study has been supported by the Deputy of Research and Technology, Yasuj University of Medical Sciences.
Authors' Contribution: Zareifrar N (First Author), Main Researcher/Introduction Writer/Discussion Writer (15%); Sharifi A (Second Author), Main Researcher/Introduction Writer/Discussion Writer/Methodologist (25%); Khosravani SA (Third Author), Methodologist/Assistant Researcher/Statistical Analyst (15%); Zoladl M (Fourth Author), Assistant Researcher/Statistical Analyst/Discussion Writer (20%); Khoramrooz SS (Fifth Author), Methodologist/Assistant Researcher/Statistical Analyst (15%); Sharifi K (Sixth Author), Assistant Researcher/Introduction Writer (10%)
Funding/Support: This article is a part of Mis. Niloofar Zareifrar MSc thesis. This study has been supported by Yasuj University of Medical Sciences, Deputy of Research and Technology.
Keywords:
References
1. Mouajou V, Adams K, DeLisle G, Quach C. Hand hygiene compliance in the prevention of hospital-acquired infections: A systematic review. J Hosp Infect. 2022;119:33-48. [Link] [DOI:10.1016/j.jhin.2021.09.016]
2. Aljamali NM, Al Najim MM. Review in hospital-acquired infection. Int J Adv Eng Res. 2020;20(III):7-20. [Link]
3. Brinkwirth S, Ayobami O, Eckmanns T, Markwart R. Hospital-acquired infections caused by enterococci: A systematic review and meta-analysis, WHO European Region, 1 January 2010 to 4 February 2020. Euro Surveill. 2021;26(45):2001628. [Link] [DOI:10.2807/1560-7917.ES.2021.26.45.2001628]
4. Massart N, Mansour A, Ross JT, Piau C, Verhoye JP, Tattevin P, et al. Mortality due to hospital-acquired infection after cardiac surgery. J Thorac Cardiovasc Surg. 2022;163(6):2131-40. [Link] [DOI:10.1016/j.jtcvs.2020.08.094]
5. Liu JY, Dickter JK. Nosocomial infections: A history of hospital-acquired infections. Gastrointest Endosc Clin N Am. 2020;30(4):637-52. [Link] [DOI:10.1016/j.giec.2020.06.001]
6. Patil RK, Kabera B, Muia CK, Ale BM. Hospital acquired infections in a private paediatric hospital in Kenya: A retrospective cross-sectional study. Pan Afr Med J. 2022;41:28. [Link] [DOI:10.11604/pamj.2022.41.28.25820]
7. Gandasasmita N, Li J, Loane DJ, Semple BD. Experimental models of hospital-acquired infections after traumatic brain injury: Challenges and opportunities. J Neurotrauma. 2024;41(7-8):752-70. [Link] [DOI:10.1089/neu.2023.0453]
8. Hu LQ, Wang J, Huang A, Wang D, Wang J. COVID-19 and improved prevention of hospital-acquired infection. Br J Anaesth. 2020;125(3):e318-9. [Link] [DOI:10.1016/j.bja.2020.05.037]
9. Abban MK, Ayerakwa EA, Mosi L, Isawumi A. The burden of hospital acquired infections and antimicrobial resistance. Heliyon. 2023;9(10):e20561. [Link] [DOI:10.1016/j.heliyon.2023.e20561]
10. Majlander J, Anttila VJ, Nurmi W, Seppälä A, Tiedje J, Muziasari W. Routine wastewater-based monitoring of antibiotic resistance in two Finnish hospitals: Focus on carbapenem resistance genes and genes associated with bacteria causing hospital-acquired infections. J Hosp Infect. 2021;117:157-64. [Link] [DOI:10.1016/j.jhin.2021.09.008]
11. Idrees M, Sawant S, Karodia N, Rahman A. Staphylococcus aureus Biofilm: Morphology, genetics, pathogenesis and treatment strategies. Int J Environ Res Public Health. 2021;18(14):7602. [Link] [DOI:10.3390/ijerph18147602]
12. Tuon FF, Suss PH, Telles JP, Dantas LR, Borges NH, Ribeiro VST. Antimicrobial treatment of staphylococcus aureus Biofilms. Antibiotics. 2023;12(1):87. [Link] [DOI:10.3390/antibiotics12010087]
13. Hair BB, Conley ME, Wienclaw TM, Conley MJ, Heaton MJ, Berges BK. Synergistic activity of silver nanoparticles and vancomycin against a spectrum of Staphylococcus aureus biofilm types. J Bacteriol Mycol. 2018;5(9):1089. [Link] [DOI:10.1101/337436]
14. Nourbakhsh F, Namvar AE. Detection of genes involved in biofilm formation in Staphylococcus aureus isolates. GMS Hyg Infect Control. 2016;11:Doc07. [Link]
15. Nandhini P, Kumar P, Mickymaray S, Alothaim AS, Somasundaram J, Rajan M. Recent developments in methicillin-resistant staphylococcus aureus (MRSA) treatment: A review. Antibiotics. 2022;11(5):606. [Link] [DOI:10.3390/antibiotics11050606]
16. Anpalagan K, Dotel R, MacFadden DR, Smith S, Voss L, Petersiel N, et al. Does adjunctive clindamycin have a role in Staphylococcus aureus bacteremia? A protocol for the adjunctive treatment domain of the Staphylococcus aureus Network Adaptive Platform (SNAP) randomized controlled trial. Clin Infect Dis. 2024;79(3):626-34. [Link] [DOI:10.1093/cid/ciae289]
17. Armengol Álvarez L, Van De Sijpe G, Desmet S, Metsemakers WJ, Spriet I, Allegaert K, et al. Ways to improve insights into clindamycin pharmacology and pharmacokinetics tailored to practice. Antibiotics. 2022;11(5):701. [Link] [DOI:10.3390/antibiotics11050701]
18. Thapa D, Pyakurel S, Thapa S, Lamsal S, Chaudhari M, Adhikari N, et al. Staphylococcus aureus with inducible clindamycin resistance and methicillin resistance in a tertiary hospital in Nepal. Trop Med Health. 2021;49(1):99. [Link] [DOI:10.1186/s41182-021-00392-2]
19. Moghadam Fard A, Nikbakht T, Babaei N, Pouyamanesh M, Afzalian A, Kharazmkia A, et al. Role of medicinal plants in treatment of inflammatory diseases. Kindle. 2022;2(1):1-139. [Link]
20. Ahluwalia O, Singh PC, Bhatia R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour Environ Sustain. 2021;5:100032. [Link] [DOI:10.1016/j.resenv.2021.100032]
21. Alesiani D, Canini A, D'Abrosca B, DellaGreca M, Fiorentino A, Mastellone C, et al. Antioxidant and antiproliferative activities of phytochemicals from Quince (Cydonia vulgaris) peels. Food Chem. 2010;118(2):199-207. [Link] [DOI:10.1016/j.foodchem.2009.04.098]
22. Al-Snafi AE. The medical importance of Cydonia oblonga-A review. IOSR J Pharm. 2016;6(6):87-99. [Link]
23. Al-Khazraji SK. Phytochemical screening and antibacterial activity of the crude extract of Cydonia oblonga seeds. Glob Adv Res J Microbiol. 2013;2(8):137-40. [Link] [DOI:10.12816/0010090]
24. Southwell IA, Bourke CA. Seasonal variation in hypericin content of Hypericum perforatum L. (St. John's Wort). Phytochemistry. 2001;56(5):437-41. [Link] [DOI:10.1016/S0031-9422(00)00411-8]
25. Nazlı O, Baygar T, Demirci Dönmez ÇE, Dere Ö, Uysal Aİ, Aksözek A, et al. Antimicrobial and antibiofilm activity of polyurethane/Hypericum perforatum extract (PHPE) composite. Bioorg Chem. 2019;82:224-8. [Link] [DOI:10.1016/j.bioorg.2018.08.017]
26. Barnes J, Anderson LA, Phillipson JD. St John's wort (Hypericum perforatum L.): A review of its chemistry, pharmacology and clinical properties. J Pharm Pharmacol. 2001;53(5):583-600. [Link] [DOI:10.1211/0022357011775910]
27. Saddiqe Z, Naeem I, Maimoona A. A review of the antibacterial activity of Hypericum perforatum L. J Ethnopharmacol. 2010;131(3):511-21. [Link] [DOI:10.1016/j.jep.2010.07.034]
28. Akhbari M, Ebrahimian M. Comparison of hypericin content, antioxidant, antimicrobial and cytotoxic activities of Hypericum perforatum L. from three geographic regions of Iran. J Gorgan Univ Med Sci. 2017;19(1):89-95. [Persian] [Link]
29. Sadeghi H, Hosseinzadeh S, Akbartabar Touri M, Ghavamzadeh M, Jafari Barmak M, Sayahi M, et al. Hepatoprotective effect of Rosa canina fruit extract against carbon tetrachloride induced hepatotoxicity in rat. Avicenna J Phytomed. 2016;6(2):181-8. [Link]
30. Abbaszadegan A, Gholami A, Ghahramani Y, Ghareghan R, Ghareghan M, Kazemi A, et al. Antimicrobial and cytotoxic activity of Cuminum cyminum as an intracanal medicament compared to chlorhexidine gel. Iran Endod J. 2016;11(1):44-50. [Link] [DOI:10.5395/rde.2015.40.1.50]
31. Koohsari H, Ghaemi EA, Sadegh Sheshpoli M, Jahedi M, Zahiri M. The investigation of antibacterial activity of selected native plants from North of Iran. J Med Life. 2015;8(Spec Iss 2):38-42. [Link]
32. Rothenburger S, Spangler D, Bhende S, Burkley D. In vitro antimicrobial evaluation of Coated VICRYL* Plus Antibacterial Suture (coated polyglactin 910 with triclosan) using zone of inhibition assays. Surg Infect. 2002;3(Suppl 1):S79-87. [Link] [DOI:10.1089/sur.2002.3.s1-79]
33. Tille P. Bailey & Scott's diagnostic microbiology. London: Elsevier Health Sciences; 2015. [Link]
34. Demir C, Demirci M, Yigin A, Tokman HB, Cetik Yildiz S. Presence of biofilm and adhesin genes in Staphylococcus aureus strains taken from chronic wound infections and their genotypic and phenotypic antimicrobial sensitivity patterns. Photodiagnosis Photodyn Ther. 2020;29:101584. [Link] [DOI:10.1016/j.pdpdt.2019.101584]
35. Yazdani R, Oshaghi M, Havayi A, Pishva E, Salehi R, Sadeghizadeh M, et al. Detection of icaAD gene and biofilm formation in Staphylococcus aureus isolates from wound infections. Iran J Public Health. 2006;35(2):25-8. [Link]
36. Dastgheyb S, Parvizi J, Shapiro IM, Hickok NJ, Otto M. Effect of biofilms on recalcitrance of staphylococcal joint infection to antibiotic treatment. J Infect Dis. 2015;211(4):641-50. [Link] [DOI:10.1093/infdis/jiu514]
37. Wang L, Yu F, Yang L, Li Q, Zhang X, Zeng Y, et al. Prevalence of virulence genes and biofilm formation among Staphylococcus aureus clinical isolates associated with lower respiratory infection. Afr J Microbiol Res. 2010;4(23):2566-9. [Link]
38. Coutinho Vde L, Paiva RM, Reiter KC, De-Paris F, Barth AL, Machado AB. Distribution of erm genes and low prevalence of inducible resistance to clindamycin among staphylococci isolates. Braz J Infect Dis. 2010;14(6):564-8. [Link] [DOI:10.1590/S1413-86702010000600004]
39. Mollaei M, Rashki A. The prevalence of adhesive surface encoding genes in staphylococcus aureus isolated from hospitalized patients in Zabol-Iran by multiplex PCR. J Adv Biomed Sci. 2016;6(3):296-302. [Persian] [Link]
40. Aygül A, Şerbetçi T. The antibacterial and antivirulent potential of Hypericum lydium against Staphylococcus aureus: Inhibition of growth, biofilm formation, and hemolytic activity. Eur J Integr Med. 2020;35:101061. [Link] [DOI:10.1016/j.eujim.2020.101061]
41. Bahmani M, Taherikalani M, Khaksarian M, Rafieian-Kopaei M, Ashrafi B, Nazer M, et al. The synergistic effect of hydroalcoholic extracts of Origanum vulgare, Hypericum perforatum and their active components carvacrol and hypericin against Staphylococcus aureus. Future Sci OA. 2019;5(3):FSO371. [Link] [DOI:10.4155/fsoa-2018-0096]








