Volume 6, Issue 2 (2025)
J Clinic Care Skill 2025, 6(2): 105-111 |
Back to browse issues page
Article Type:
Subject:
Ethics code: IR.YUMS.REC.1402.037
History
Received: 2025/05/22 | Accepted: 2025/06/30 | Published: 2025/07/2
Received: 2025/05/22 | Accepted: 2025/06/30 | Published: 2025/07/2
How to cite this article
Asadikalameh Z, Golshahi Chamandani F, Sharifi M. Effect of Sildenafil on Fetal Heart Rate Pattern During Labor. J Clinic Care Skill 2025; 6 (2) :105-111
URL: http://jccs.yums.ac.ir/article-1-400-en.html
URL: http://jccs.yums.ac.ir/article-1-400-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Gynecology and Obstetrics, Faculty of Medical, Yasuj University of Medical Sciences, Yasuj, Iran
2- “Student Research Committee” and “Faculty of Medical”, Yasuj University of Medical Sciences, Yasuj, Iran
3- Department of Midwifery, Faculty of Medical, Yasuj University of Medical Sciences, Yasuj, Iran
2- “Student Research Committee” and “Faculty of Medical”, Yasuj University of Medical Sciences, Yasuj, Iran
3- Department of Midwifery, Faculty of Medical, Yasuj University of Medical Sciences, Yasuj, Iran
Full-Text (HTML) (632 Views)
Introduction
Fetal heart rate (FHR) patterns are monitored throughout pregnancy and labor to evaluate the health of the fetus. Patterns observed through electronic fetal monitoring may indicate whether a fetus is experiencing distress. Analyzing these patterns helps clinicians determine appropriate interventions, such as changing the maternal position, administering oxygen, or potentially opting for delivery [1]. FHR patterns observed during labor may signal fetal distress and the risk of birth asphyxia. In particular, specific FHR patterns, such as late decelerations, prolonged decelerations, and reduced variability, are associated with a higher risk of fetal asphyxia, a condition in which the fetus suffers from inadequate oxygen. It is essential to recognize that abnormal FHR patterns do not always signify asphyxia, and various other factors must be taken into account [2].
Fetal distress, a condition in which the fetus encounters insufficient oxygen or other issues during pregnancy or labor, may lead to various negative outcomes. These outcomes include hypoxic-ischemic encephalopathy (HIE), cerebral palsy, seizures, developmental delays, and potentially stillbirth. Immediate complications primarily include hypoxia (insufficient oxygen) and acidosis (excess acid in the blood), which can harm the brain and other organs. Chronic complications may involve cerebral palsy, developmental setbacks, learning difficulties, and various other neurological problems [3].
Averting fetal distress is vital, as it may lead to serious issues for the baby, such as brain injury, cerebral palsy, or even stillbirth if not managed quickly. By actively observing both the mother and the fetus, addressing underlying problems, and promptly intervening upon noticing signs of distress, healthcare professionals can reduce risks and promote healthy outcomes for both the mother and the child [4]. Although fetal distress cannot always be avoided, taking proactive steps such as consistent prenatal check-ups, maintaining a healthy lifestyle, and avoiding harmful substances can significantly diminish the risk. Timely identification and swift intervention for issues, such as preeclampsia or gestational diabetes, are equally important. Furthermore, monitoring fetal movement and promptly informing your healthcare provider about any unusual symptoms can aid in the early detection of potential problems [5].
Sildenafil has been proposed as a treatment to alleviate fetal distress. It encourages vasodilation by blocking the enzyme cyclic GMP phosphodiesterase type 5, thereby enabling cyclic GMP to trigger the release of nitric oxide, a potent vasodilator. Pfizer first explored sildenafil in the mid-1980s as a potential substitute for nitrates in the treatment of angina pectoris [6]. Sildenafil received market authorization from the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) in 1998. Since then, it has been approved by the FDA and EMA for both adult and pediatric pulmonary arterial hypertension and has been studied for various conditions, including Raynaud’s disease, heart failure, cardiac and cerebral ischemia, as well as infarction [7, 8]. The rationale for using sildenafil to address these issues can be understood through its ability to counter endothelial dysfunction, prevent the remodeling of vascular smooth muscle, and specifically enhance blood flow in regions with the highest demand by promoting vascular smooth muscle relaxation [9].
During pregnancy, sildenafil has demonstrated beneficial outcomes in animals [10, 11]. Research is currently underway to explore its effects on human pregnancy, and initial findings indicate promising results. For instance, two clinical studies report slightly reduced maternal blood pressure in expectant mothers with preeclampsia, with no safety concerns related to sildenafil for mothers, fetuses, or newborns [12, 13]. Another study indicated that incorporating sildenafil into nifedipine treatment for threatened preterm labor has advantageous effects, noting extended latency, a lower prevalence of neonatal respiratory distress syndrome, and increased birth weights [14]. The findings of these studies have motivated researchers in maternal health to explore the effects of sildenafil on fetal heart patterns during childbirth. A trial conducted to investigate the reduction of fetal distress with sildenafil shows a decrease in the risk of meconium-stained amniotic fluid and abnormal FHR patterns, with no differences in maternal or neonatal adverse outcomes [15]. Due to the limited number of trials on the impact of sildenafil on fetal distress, additional evidence-based findings are required to gain more insights into its effects on alleviating fetal distress during labor. In this study, we sought to assess the effect of sildenafil on the FHR pattern during labor.
Materials and Methods
Study design and Participants
This single-blind randomized controlled trial was conducted in the maternity unit of Imam Sajjad Hospital in Yasuj in 2024. The study population consisted of primiparous pregnant women who were referred to Yasuj Hospital and met the inclusion criteria.
Participants were primiparous, had a full-term pregnancy (gestational age exceeding 37 weeks), and presented with a single pregnancy in cephalic presentation. Additionally, they had no cesarean indications at the time of allocation and serious systemic or chronic diseases, such as diabetes, hypertension, autoimmune diseases, or anemia (with hemoglobin less than 10g/dL). Furthermore, they had no known fetal anomalies, fetal growth restriction (defined as an estimated fetal weight or abdominal circumference (AC) below the 10th percentile for gestational age), polyhydramnios (defined as an amniotic fluid index (AFI) of 24cm or a deepest vertical pocket (DVP) between 8 and 11cm), or oligohydramnios (AFI less than 5cm or DVP less than 2cm). Cervical dilation was less than 4cm at the time of admission, and the amniotic fluid was clear at the time of allocation. Participants were excluded if they had a prolonged rupture of membranes (exceeding 12 hours) and if magnesium sulfate was administered during labor.
Taking into account the study by Turner et al. [15], a cesarean section rate of 18% in the intervention group and 36.7% in the control group, with a type I error of 0.05 and a type II error of 0.20, the total sample size was projected to consist of 100 individuals in each group using the following formula.
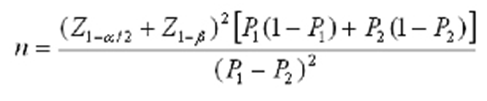
Procedure
The approach to randomization was straightforward. The groups were coded as follows: “A” for the sildenafil intervention group and “B” for the control group. The randomization results for the 200 women were printed in a Word document by a faculty member who was not involved in the study. Papers coded “A” or “B” were placed in opaque envelopes. During the data collection phase, the assigned individual conducted interviews with the women, assessed the inclusion criteria, and obtained informed consent from the participants before allocation. The women were asked to select an envelope. For example, a woman who selected the envelope with the code “A” was assigned to the sildenafil intervention group, while a woman who selected the envelope with the code “B” was assigned to the control group. To prevent bias and ensure confidentiality during randomization, it was conducted by a statistical expert who was not one of the authors.
This clinical research is registered with the Iranian Registry of Clinical Trials (IRCT20160524028038N19; https://irct.behdasht.gov.ir/trial/75672). The reporting adhered to the CONSORT extension for parallel group randomized trials (CONSORT 2010 Flow Diagram) and the CONSORT 2010 Checklist [16]. The study’s objectives were explained to the participants, and written informed consent was obtained from the eligible mothers to participate in the study. The mothers were informed that their private information would be kept confidential and that names, along with personal details, would not be used at any point during the study. Furthermore, the mothers did not incur any additional costs at any point during the study. All stages of the study were conducted in accordance with the ethical principles outlined in the Declaration of Helsinki.
Intervention
The intervention group received oral sildenafil at a dose of 50mg every 8 hours, with a maximum of 150mg, starting from the first dose when the study began. The control group received only the usual standard care during labor. We checked the mothers’ blood pressure approximately 15 to 30 minutes after each dose. The FHR was monitored and recorded continuously throughout the study. Standard labor care included observing maternal uterine contractions, checking vital signs, conducting vaginal examinations, and tracking labor progress using a Partograph.
Outcome measures
The reassuring FHR pattern established by the standard protocol was defined as a heart rate of 110 to 160bpm with a variability of 5 to 25bpm from one beat to the next during labor. A variable deceleration (a rapid decline in FHR of 15bpm or more lasting at least 15 seconds before the heart rate returns to normal), an early deceleration (a symmetrical decline and rise in FHR linked to a uterine contraction), a late deceleration (a noticeable, gradual decrease in FHR usually following a uterine contraction), reduced variability (less than 5bpm), tachycardia (FHR exceeding 160bpm), and bradycardia (FHR below 110bpm) were all identified as pathological and non-reassuring fetal heart patterns [17]. We assessed labor duration, meconium-stained amniotic fluid, delivery method, and postpartum hemorrhage (blood loss of ≥500ml, measured visually), as well as neonatal outcomes, including the Apgar score of the newborn and admission to the neonatal intensive care unit (NICU). Adverse effects were also considered outcome measures. We employed a checklist and questionnaire to gather data.
Data analysis
The data were analyzed using SPSS 21. Initially, the distribution of quantitative data was assessed; normal data were assessed using the t-test, whereas data with no normal distribution were assessed using the Mann-Whitney U test. The significance level was set at 0.05. Chi-square tests for proportion comparison were employed to analyze qualitative data.
Findings
We screened 252 cases according to the eligibility criteria, of which 50 cases were excluded (41 cases did not meet the inclusion criteria, and 9 cases declined to participate). A total of 202 cases met the inclusion criteria and were randomly divided into two groups (Figure 1). During the study, some cases in the intervention group discontinued participation, and ultimately, 96 cases in the sildenafil group and 102 cases in the control group were included in the analysis.
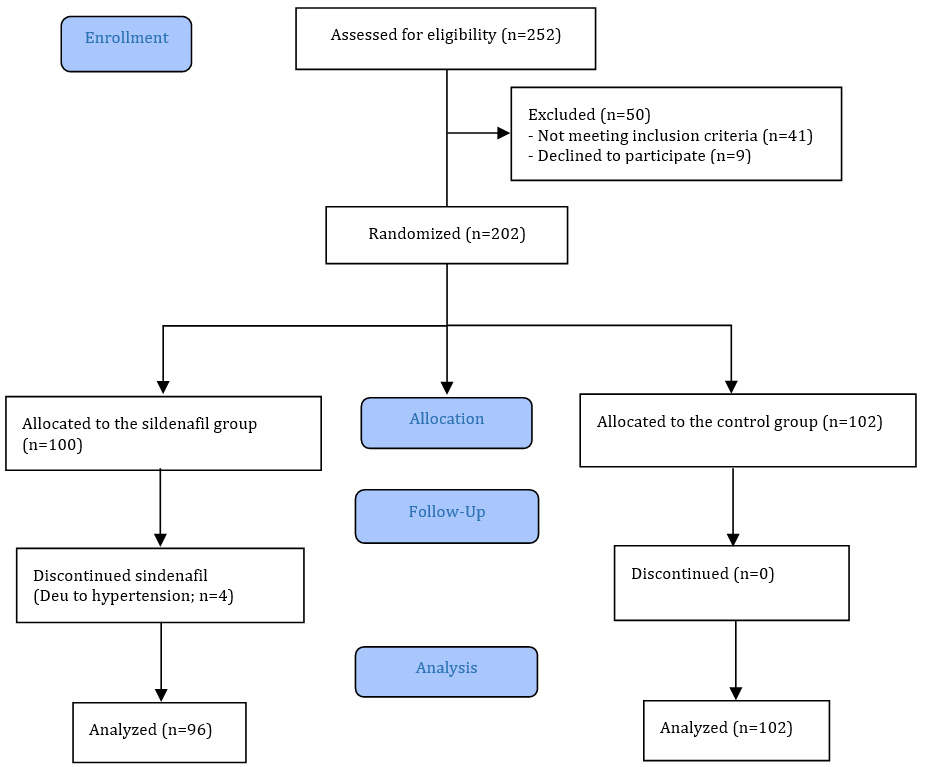
Figure 1. CONSORT 2010 flow diagram
The two groups differed in terms of the occupations of the mothers, with mothers working full-time being more prevalent in the control group. The mean age of mothers in the Intervention group was 25.38±5.81 years, while in the Control group it was 26.13±6.58 years (p=0.204). Additionally, the mean gestational age in the Intervention group was 271.98±6.18 days, whereas in the Control group it was 273.82±7.52 days (p=0.013; Table 1).
Table 1. Comparison of frequency of demographic and maternal factors between groups
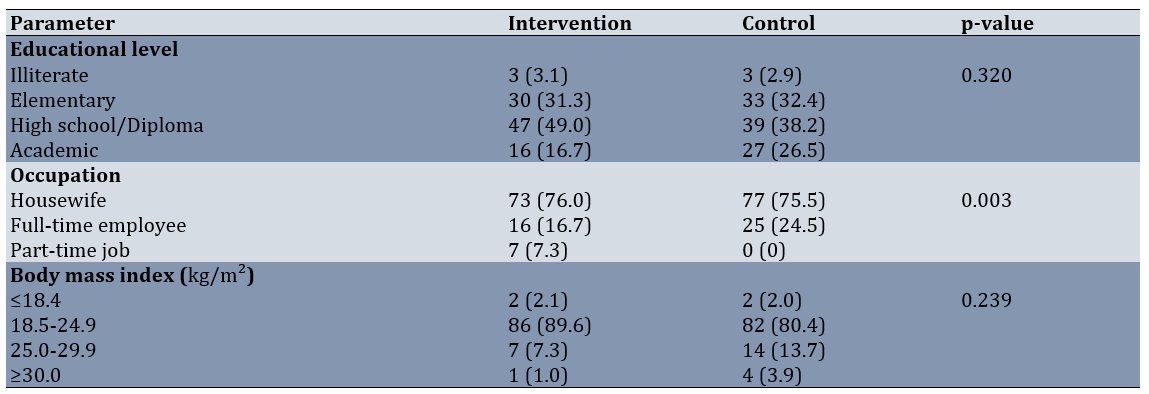
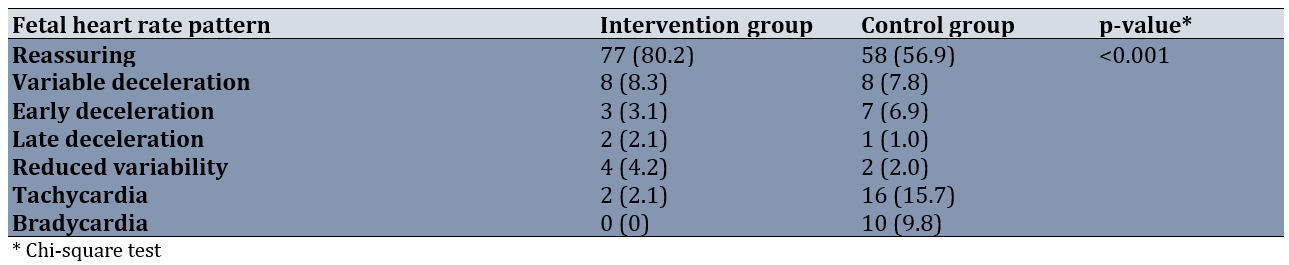
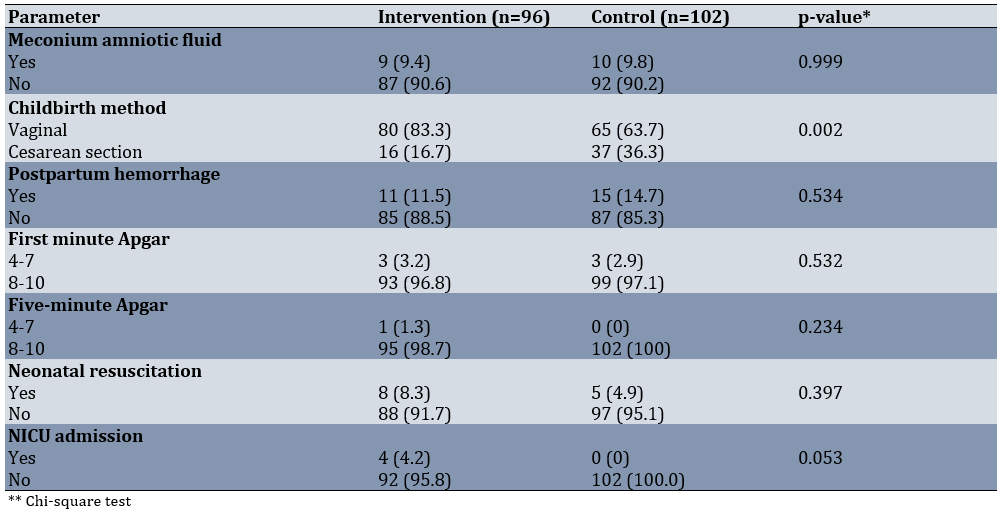
The reassuring fetal heart pattern was more common in the sildenafil group (80.2% compared to 56.9%). Among the various non-reassuring fetal heart patterns, tachycardia and bradycardia were the most prevalent in the control group compared to the sildenafil group (Table 2).
Table 2. Comparison of the frequency of fetal heart rate patterns between groups
We analyzed the outcomes for mothers and newborns in both groups. Duration of labor was 25.38±5.81 and 26.13±6.58 hours in the intervention and control groups, respectively (p=0.204). Cesarean deliveries occurred more frequently in the control group than in the sildenafil group (36.3% vs. 16.7%). The difference in postpartum hemorrhage rates between the groups was not statistically significant. Neonatal outcomes, such as Apgar scores and the rate of neonatal resuscitation, were comparable in both groups. The NICU admission rate was higher in the sildenafil group than in the control group, but this difference was not statistically significant (Table 3).
Table 3. Comparison of the frequency of maternal and neonatal outcomes between groups
Discussion
This study assessed the effect of sildenafil on the FHR pattern during labor. The global health issue of perinatal mortality and morbidity linked to fetal hypoxia during labor remains significant. Monitoring the fetus during labor is vital for preventing birth asphyxia, a situation in which a newborn fails to receive adequate oxygen during delivery, potentially resulting in brain injury or death. Ongoing or intermittent observation of FHR and uterine contractions, typically using cardiotocography, aids in recognizing indicators of fetal distress, facilitating prompt action to avert asphyxia [4].
When the fetus experiences distress, several actions can be taken, such as resuscitation, positioning the mother laterally on her left side, and providing oxygen to the mother. If she is receiving oxytocin, we discontinue it, provide hydration to the mother, and observe the fetus. If the situation does not improve, the physician may decide to perform an emergency cesarean section [2, 5]. Currently, the only options for addressing this condition are emergency cesarean section or the use of devices to accelerate the labor process. Research has indicated positive outcomes from using sildenafil citrate to enhance uteroplacental blood circulation and, consequently, fetal oxygenation [11, 12]. Sildenafil, which is used to treat men with erectile dysfunction (often referred to as sexual impotence), belongs to a class of medications known as phosphodiesterase 5 (PDE5) inhibitors. These drugs inhibit an enzyme known as phosphodiesterase type 5 from acting too rapidly [6, 7]. Sildenafil enhances uteroplacental blood circulation through vasodilation and has demonstrated improvements in perinatal outcomes in cases of fetal growth restriction or pregnancy-related preeclampsia [13]. It may also serve as a potential treatment method to improve uteroplacental blood circulation during pregnancy. Recently, research has been conducted on sildenafil’s impact during labor to decrease the chances of fetal distress, and the findings have been promising [14].
In research conducted by Turner et al., women in the early stages of labor or those undergoing scheduled labor induction at the Maternity Hospital in Brisbane, Australia, were randomly allocated to receive either 50mg of oral sildenafil citrate every 8 hours, 150mg, or a placebo. According to their findings, sildenafil decreases the likelihood of emergency cesarean delivery by 51%. No differences are observed in the indices of fetal and uteroplacental perfusion, although these indices are assessed in only 71 women. Sildenafil citrate reduces the likelihood of meconium-stained amniotic fluid or abnormal FHR patterns by 43%, yet its impact on the incidence of fetal scalp biopsy and negative neonatal outcomes remains unclear [15].
We examined the impact of sildenafil on alleviating fetal distress during labor in expectant mothers. The reassuring FHR pattern occurred more frequently in the sildenafil group (80.2% versus 56.9%). Among the various non-reassuring FHR patterns, tachycardia and bradycardia were the most common patterns in the control group compared to the sildenafil group. Cesarean deliveries were more frequent in the control group than in the sildenafil group. The neonatal outcomes were comparable in both groups, and no side effects were reported.
The frequency of a reassuring FHR pattern in individuals who used sildenafil was greater than that in the control group. Subgroup analysis of pathological FHR patterns among the groups indicated that early decelerations, fetal tachycardia, and bradycardia were less common in the sildenafil group compared to the control group, although late and variable decelerations did not show statistically significant differences. It has been suggested that sildenafil can increase uterine blood flow [18]. Uterine perfusion plays a crucial role in determining the FHR pattern. Reduced uterine blood flow is frequently associated with fetal growth restriction [19], decreased birth weights [20], higher levels of fetal distress [21], as well as elevated rates of fetal mortality and neonatal illness and mortality. Thus, any medication that improves uterine blood flow could be beneficial in decreasing negative outcomes for neonates.
Although fetal distress was lower in the sildenafil group than in the control group, the rates of neonatal resuscitation and NICU admissions were higher in the sildenafil group; however, the differences were not statistically significant. Considering that fetal distress is a prevalent issue during labor that can lead to outcomes, such as emergency cesarean deliveries or a higher demand for neonatal assistance [22, 23], it is anticipated that the findings of this study will provide a definitive approach for using oral sildenafil to alleviate fetal distress, thereby decreasing the incidence of emergency cesarean sections and their associated effects, as well as minimizing neonatal interventions.
Identifying the risk factors associated with fetal distress [24, 25] and creating various interventions to reduce it is a goal of the midwifery and women’s health team to prevent adverse neonatal outcomes and lower the cesarean section rate attributable to fetal distress [4, 26]. Our results can contribute to the literature in alignment with this stated objective.
However, in contrast to our findings, a randomized trial with a placebo control involving 3,257 women demonstrates no difference in the incidence of the main composite outcome of infant mortality or neonatal morbidity when comparing women given oral sildenafil citrate in labor to those receiving a placebo [27].
A systematic review of sildenafil administration during pregnancy, particularly for fetal growth restriction (FGR) and preeclampsia, indicates possible advantages, such as enhanced fetal growth and regulation of maternal blood pressure. Nevertheless, the review also emphasizes potential dangers, including an increased risk of persistent pulmonary hypertension of the newborn (PPHN) when used for FGR management. Sildenafil is typically not recommended during pregnancy and should only be used under close medical supervision if deemed essential. It has also been reported to cause side effects, such as headache, visual disturbances, dyspepsia/epigastric pain, and hypotension [28].
Electronic FHR monitoring is a commonly used method for evaluating fetal condition during labor. Although there is limited evidence regarding its effectiveness, this method remains widely utilized in all contemporary labor and delivery units in developed nations. All healthcare providers attending to the laboring woman and her newborn must have a solid understanding of the fundamental pathophysiology of FHR monitoring and recognize the progression of labor, along with any concerns that arise, to enhance outcomes and ensure patient safety [4, 5]. Sildenafil use, due to its vasodilatory effect, decreased the occurrence of fetal distress during labor, resulting in fewer fetal complications and a lower rate of emergency cesarean deliveries. Consequently, this medication may serve as an appropriate option and can be considered a suggested treatment to alleviate fetal distress and reduce cesarean deliveries.
This study, while demonstrating encouraging outcomes regarding sildenafil’s effectiveness in alleviating fetal distress, has several limitations that we recommend other researchers consider in future investigations. First, some demographic factors, such as residency location [29, 30], may influence childbirth outcomes and were not taken into account in this research. The effect of doula presence during labor was also not considered a confounding factor in this study. Small sample sizes, due to limited patient populations or the study of rare diseases, continue to pose challenges in clinical trials. Reduced sample sizes may lower statistical power, hinder the identification of actual treatment effects, and increase the likelihood of false negatives (Type II errors). This may obstruct the reliability and reproducibility of research results.
The administration of sildenafil demonstrated promising effects in mitigating non-reassuring FHR patterns, particularly tachycardia and bradycardia, and contributed to a reduction in the rate of cesarean deliveries. Despite these benefits, neonatal outcomes remained comparable to those observed in the control group. These findings suggest potential relevance for future clinical investigations of sildenafil citrate in human populations. However, further studies involving larger cohorts are warranted to strengthen the evidence and allow for more definitive conclusions. In designing subsequent trials, the aforementioned limitations should be carefully considered.
Conclusion
Sildenafil improves non-reassuring FHR patterns, especially tachycardia and bradycardia, and decreases the rate of cesarean deliveries.
Acknowledgments: We acknowledge Yasuj University of Medical Sciences for providing research funding.
Ethical Permissions: The ethical guidelines for research and the required approvals were obtained from the vice-chancellor of research at Yasuj University of Medical Sciences (IR.YUMS.REC.1402.037).
Conflicts of Interests: The authors declared that they have no competing interests.
Authors' Contribution: Asadikalameh Z (First Author), Methodologist/Main Researcher/Discussion Writer (40%); Golshahi Chamandani F (Second Author), Introduction Writer/Assistant Researcher (20%); Sharifi M (Third Author), Methodologist/Assistant Researcher/Discussion Writer/Statistical Analyst (40%)
Funding/Support: The research received funding from Yasuj University of Medical Sciences.
Fetal heart rate (FHR) patterns are monitored throughout pregnancy and labor to evaluate the health of the fetus. Patterns observed through electronic fetal monitoring may indicate whether a fetus is experiencing distress. Analyzing these patterns helps clinicians determine appropriate interventions, such as changing the maternal position, administering oxygen, or potentially opting for delivery [1]. FHR patterns observed during labor may signal fetal distress and the risk of birth asphyxia. In particular, specific FHR patterns, such as late decelerations, prolonged decelerations, and reduced variability, are associated with a higher risk of fetal asphyxia, a condition in which the fetus suffers from inadequate oxygen. It is essential to recognize that abnormal FHR patterns do not always signify asphyxia, and various other factors must be taken into account [2].
Fetal distress, a condition in which the fetus encounters insufficient oxygen or other issues during pregnancy or labor, may lead to various negative outcomes. These outcomes include hypoxic-ischemic encephalopathy (HIE), cerebral palsy, seizures, developmental delays, and potentially stillbirth. Immediate complications primarily include hypoxia (insufficient oxygen) and acidosis (excess acid in the blood), which can harm the brain and other organs. Chronic complications may involve cerebral palsy, developmental setbacks, learning difficulties, and various other neurological problems [3].
Averting fetal distress is vital, as it may lead to serious issues for the baby, such as brain injury, cerebral palsy, or even stillbirth if not managed quickly. By actively observing both the mother and the fetus, addressing underlying problems, and promptly intervening upon noticing signs of distress, healthcare professionals can reduce risks and promote healthy outcomes for both the mother and the child [4]. Although fetal distress cannot always be avoided, taking proactive steps such as consistent prenatal check-ups, maintaining a healthy lifestyle, and avoiding harmful substances can significantly diminish the risk. Timely identification and swift intervention for issues, such as preeclampsia or gestational diabetes, are equally important. Furthermore, monitoring fetal movement and promptly informing your healthcare provider about any unusual symptoms can aid in the early detection of potential problems [5].
Sildenafil has been proposed as a treatment to alleviate fetal distress. It encourages vasodilation by blocking the enzyme cyclic GMP phosphodiesterase type 5, thereby enabling cyclic GMP to trigger the release of nitric oxide, a potent vasodilator. Pfizer first explored sildenafil in the mid-1980s as a potential substitute for nitrates in the treatment of angina pectoris [6]. Sildenafil received market authorization from the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) in 1998. Since then, it has been approved by the FDA and EMA for both adult and pediatric pulmonary arterial hypertension and has been studied for various conditions, including Raynaud’s disease, heart failure, cardiac and cerebral ischemia, as well as infarction [7, 8]. The rationale for using sildenafil to address these issues can be understood through its ability to counter endothelial dysfunction, prevent the remodeling of vascular smooth muscle, and specifically enhance blood flow in regions with the highest demand by promoting vascular smooth muscle relaxation [9].
During pregnancy, sildenafil has demonstrated beneficial outcomes in animals [10, 11]. Research is currently underway to explore its effects on human pregnancy, and initial findings indicate promising results. For instance, two clinical studies report slightly reduced maternal blood pressure in expectant mothers with preeclampsia, with no safety concerns related to sildenafil for mothers, fetuses, or newborns [12, 13]. Another study indicated that incorporating sildenafil into nifedipine treatment for threatened preterm labor has advantageous effects, noting extended latency, a lower prevalence of neonatal respiratory distress syndrome, and increased birth weights [14]. The findings of these studies have motivated researchers in maternal health to explore the effects of sildenafil on fetal heart patterns during childbirth. A trial conducted to investigate the reduction of fetal distress with sildenafil shows a decrease in the risk of meconium-stained amniotic fluid and abnormal FHR patterns, with no differences in maternal or neonatal adverse outcomes [15]. Due to the limited number of trials on the impact of sildenafil on fetal distress, additional evidence-based findings are required to gain more insights into its effects on alleviating fetal distress during labor. In this study, we sought to assess the effect of sildenafil on the FHR pattern during labor.
Materials and Methods
Study design and Participants
This single-blind randomized controlled trial was conducted in the maternity unit of Imam Sajjad Hospital in Yasuj in 2024. The study population consisted of primiparous pregnant women who were referred to Yasuj Hospital and met the inclusion criteria.
Participants were primiparous, had a full-term pregnancy (gestational age exceeding 37 weeks), and presented with a single pregnancy in cephalic presentation. Additionally, they had no cesarean indications at the time of allocation and serious systemic or chronic diseases, such as diabetes, hypertension, autoimmune diseases, or anemia (with hemoglobin less than 10g/dL). Furthermore, they had no known fetal anomalies, fetal growth restriction (defined as an estimated fetal weight or abdominal circumference (AC) below the 10th percentile for gestational age), polyhydramnios (defined as an amniotic fluid index (AFI) of 24cm or a deepest vertical pocket (DVP) between 8 and 11cm), or oligohydramnios (AFI less than 5cm or DVP less than 2cm). Cervical dilation was less than 4cm at the time of admission, and the amniotic fluid was clear at the time of allocation. Participants were excluded if they had a prolonged rupture of membranes (exceeding 12 hours) and if magnesium sulfate was administered during labor.
Taking into account the study by Turner et al. [15], a cesarean section rate of 18% in the intervention group and 36.7% in the control group, with a type I error of 0.05 and a type II error of 0.20, the total sample size was projected to consist of 100 individuals in each group using the following formula.
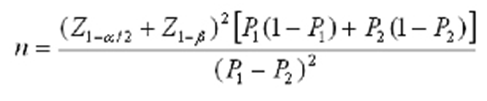
Procedure
The approach to randomization was straightforward. The groups were coded as follows: “A” for the sildenafil intervention group and “B” for the control group. The randomization results for the 200 women were printed in a Word document by a faculty member who was not involved in the study. Papers coded “A” or “B” were placed in opaque envelopes. During the data collection phase, the assigned individual conducted interviews with the women, assessed the inclusion criteria, and obtained informed consent from the participants before allocation. The women were asked to select an envelope. For example, a woman who selected the envelope with the code “A” was assigned to the sildenafil intervention group, while a woman who selected the envelope with the code “B” was assigned to the control group. To prevent bias and ensure confidentiality during randomization, it was conducted by a statistical expert who was not one of the authors.
This clinical research is registered with the Iranian Registry of Clinical Trials (IRCT20160524028038N19; https://irct.behdasht.gov.ir/trial/75672). The reporting adhered to the CONSORT extension for parallel group randomized trials (CONSORT 2010 Flow Diagram) and the CONSORT 2010 Checklist [16]. The study’s objectives were explained to the participants, and written informed consent was obtained from the eligible mothers to participate in the study. The mothers were informed that their private information would be kept confidential and that names, along with personal details, would not be used at any point during the study. Furthermore, the mothers did not incur any additional costs at any point during the study. All stages of the study were conducted in accordance with the ethical principles outlined in the Declaration of Helsinki.
Intervention
The intervention group received oral sildenafil at a dose of 50mg every 8 hours, with a maximum of 150mg, starting from the first dose when the study began. The control group received only the usual standard care during labor. We checked the mothers’ blood pressure approximately 15 to 30 minutes after each dose. The FHR was monitored and recorded continuously throughout the study. Standard labor care included observing maternal uterine contractions, checking vital signs, conducting vaginal examinations, and tracking labor progress using a Partograph.
Outcome measures
The reassuring FHR pattern established by the standard protocol was defined as a heart rate of 110 to 160bpm with a variability of 5 to 25bpm from one beat to the next during labor. A variable deceleration (a rapid decline in FHR of 15bpm or more lasting at least 15 seconds before the heart rate returns to normal), an early deceleration (a symmetrical decline and rise in FHR linked to a uterine contraction), a late deceleration (a noticeable, gradual decrease in FHR usually following a uterine contraction), reduced variability (less than 5bpm), tachycardia (FHR exceeding 160bpm), and bradycardia (FHR below 110bpm) were all identified as pathological and non-reassuring fetal heart patterns [17]. We assessed labor duration, meconium-stained amniotic fluid, delivery method, and postpartum hemorrhage (blood loss of ≥500ml, measured visually), as well as neonatal outcomes, including the Apgar score of the newborn and admission to the neonatal intensive care unit (NICU). Adverse effects were also considered outcome measures. We employed a checklist and questionnaire to gather data.
Data analysis
The data were analyzed using SPSS 21. Initially, the distribution of quantitative data was assessed; normal data were assessed using the t-test, whereas data with no normal distribution were assessed using the Mann-Whitney U test. The significance level was set at 0.05. Chi-square tests for proportion comparison were employed to analyze qualitative data.
Findings
We screened 252 cases according to the eligibility criteria, of which 50 cases were excluded (41 cases did not meet the inclusion criteria, and 9 cases declined to participate). A total of 202 cases met the inclusion criteria and were randomly divided into two groups (Figure 1). During the study, some cases in the intervention group discontinued participation, and ultimately, 96 cases in the sildenafil group and 102 cases in the control group were included in the analysis.

Figure 1. CONSORT 2010 flow diagram
The two groups differed in terms of the occupations of the mothers, with mothers working full-time being more prevalent in the control group. The mean age of mothers in the Intervention group was 25.38±5.81 years, while in the Control group it was 26.13±6.58 years (p=0.204). Additionally, the mean gestational age in the Intervention group was 271.98±6.18 days, whereas in the Control group it was 273.82±7.52 days (p=0.013; Table 1).
Table 1. Comparison of frequency of demographic and maternal factors between groups

The reassuring fetal heart pattern was more common in the sildenafil group (80.2% compared to 56.9%). Among the various non-reassuring fetal heart patterns, tachycardia and bradycardia were the most prevalent in the control group compared to the sildenafil group (Table 2).
Table 2. Comparison of the frequency of fetal heart rate patterns between groups

We analyzed the outcomes for mothers and newborns in both groups. Duration of labor was 25.38±5.81 and 26.13±6.58 hours in the intervention and control groups, respectively (p=0.204). Cesarean deliveries occurred more frequently in the control group than in the sildenafil group (36.3% vs. 16.7%). The difference in postpartum hemorrhage rates between the groups was not statistically significant. Neonatal outcomes, such as Apgar scores and the rate of neonatal resuscitation, were comparable in both groups. The NICU admission rate was higher in the sildenafil group than in the control group, but this difference was not statistically significant (Table 3).
Table 3. Comparison of the frequency of maternal and neonatal outcomes between groups

Discussion
This study assessed the effect of sildenafil on the FHR pattern during labor. The global health issue of perinatal mortality and morbidity linked to fetal hypoxia during labor remains significant. Monitoring the fetus during labor is vital for preventing birth asphyxia, a situation in which a newborn fails to receive adequate oxygen during delivery, potentially resulting in brain injury or death. Ongoing or intermittent observation of FHR and uterine contractions, typically using cardiotocography, aids in recognizing indicators of fetal distress, facilitating prompt action to avert asphyxia [4].
When the fetus experiences distress, several actions can be taken, such as resuscitation, positioning the mother laterally on her left side, and providing oxygen to the mother. If she is receiving oxytocin, we discontinue it, provide hydration to the mother, and observe the fetus. If the situation does not improve, the physician may decide to perform an emergency cesarean section [2, 5]. Currently, the only options for addressing this condition are emergency cesarean section or the use of devices to accelerate the labor process. Research has indicated positive outcomes from using sildenafil citrate to enhance uteroplacental blood circulation and, consequently, fetal oxygenation [11, 12]. Sildenafil, which is used to treat men with erectile dysfunction (often referred to as sexual impotence), belongs to a class of medications known as phosphodiesterase 5 (PDE5) inhibitors. These drugs inhibit an enzyme known as phosphodiesterase type 5 from acting too rapidly [6, 7]. Sildenafil enhances uteroplacental blood circulation through vasodilation and has demonstrated improvements in perinatal outcomes in cases of fetal growth restriction or pregnancy-related preeclampsia [13]. It may also serve as a potential treatment method to improve uteroplacental blood circulation during pregnancy. Recently, research has been conducted on sildenafil’s impact during labor to decrease the chances of fetal distress, and the findings have been promising [14].
In research conducted by Turner et al., women in the early stages of labor or those undergoing scheduled labor induction at the Maternity Hospital in Brisbane, Australia, were randomly allocated to receive either 50mg of oral sildenafil citrate every 8 hours, 150mg, or a placebo. According to their findings, sildenafil decreases the likelihood of emergency cesarean delivery by 51%. No differences are observed in the indices of fetal and uteroplacental perfusion, although these indices are assessed in only 71 women. Sildenafil citrate reduces the likelihood of meconium-stained amniotic fluid or abnormal FHR patterns by 43%, yet its impact on the incidence of fetal scalp biopsy and negative neonatal outcomes remains unclear [15].
We examined the impact of sildenafil on alleviating fetal distress during labor in expectant mothers. The reassuring FHR pattern occurred more frequently in the sildenafil group (80.2% versus 56.9%). Among the various non-reassuring FHR patterns, tachycardia and bradycardia were the most common patterns in the control group compared to the sildenafil group. Cesarean deliveries were more frequent in the control group than in the sildenafil group. The neonatal outcomes were comparable in both groups, and no side effects were reported.
The frequency of a reassuring FHR pattern in individuals who used sildenafil was greater than that in the control group. Subgroup analysis of pathological FHR patterns among the groups indicated that early decelerations, fetal tachycardia, and bradycardia were less common in the sildenafil group compared to the control group, although late and variable decelerations did not show statistically significant differences. It has been suggested that sildenafil can increase uterine blood flow [18]. Uterine perfusion plays a crucial role in determining the FHR pattern. Reduced uterine blood flow is frequently associated with fetal growth restriction [19], decreased birth weights [20], higher levels of fetal distress [21], as well as elevated rates of fetal mortality and neonatal illness and mortality. Thus, any medication that improves uterine blood flow could be beneficial in decreasing negative outcomes for neonates.
Although fetal distress was lower in the sildenafil group than in the control group, the rates of neonatal resuscitation and NICU admissions were higher in the sildenafil group; however, the differences were not statistically significant. Considering that fetal distress is a prevalent issue during labor that can lead to outcomes, such as emergency cesarean deliveries or a higher demand for neonatal assistance [22, 23], it is anticipated that the findings of this study will provide a definitive approach for using oral sildenafil to alleviate fetal distress, thereby decreasing the incidence of emergency cesarean sections and their associated effects, as well as minimizing neonatal interventions.
Identifying the risk factors associated with fetal distress [24, 25] and creating various interventions to reduce it is a goal of the midwifery and women’s health team to prevent adverse neonatal outcomes and lower the cesarean section rate attributable to fetal distress [4, 26]. Our results can contribute to the literature in alignment with this stated objective.
However, in contrast to our findings, a randomized trial with a placebo control involving 3,257 women demonstrates no difference in the incidence of the main composite outcome of infant mortality or neonatal morbidity when comparing women given oral sildenafil citrate in labor to those receiving a placebo [27].
A systematic review of sildenafil administration during pregnancy, particularly for fetal growth restriction (FGR) and preeclampsia, indicates possible advantages, such as enhanced fetal growth and regulation of maternal blood pressure. Nevertheless, the review also emphasizes potential dangers, including an increased risk of persistent pulmonary hypertension of the newborn (PPHN) when used for FGR management. Sildenafil is typically not recommended during pregnancy and should only be used under close medical supervision if deemed essential. It has also been reported to cause side effects, such as headache, visual disturbances, dyspepsia/epigastric pain, and hypotension [28].
Electronic FHR monitoring is a commonly used method for evaluating fetal condition during labor. Although there is limited evidence regarding its effectiveness, this method remains widely utilized in all contemporary labor and delivery units in developed nations. All healthcare providers attending to the laboring woman and her newborn must have a solid understanding of the fundamental pathophysiology of FHR monitoring and recognize the progression of labor, along with any concerns that arise, to enhance outcomes and ensure patient safety [4, 5]. Sildenafil use, due to its vasodilatory effect, decreased the occurrence of fetal distress during labor, resulting in fewer fetal complications and a lower rate of emergency cesarean deliveries. Consequently, this medication may serve as an appropriate option and can be considered a suggested treatment to alleviate fetal distress and reduce cesarean deliveries.
This study, while demonstrating encouraging outcomes regarding sildenafil’s effectiveness in alleviating fetal distress, has several limitations that we recommend other researchers consider in future investigations. First, some demographic factors, such as residency location [29, 30], may influence childbirth outcomes and were not taken into account in this research. The effect of doula presence during labor was also not considered a confounding factor in this study. Small sample sizes, due to limited patient populations or the study of rare diseases, continue to pose challenges in clinical trials. Reduced sample sizes may lower statistical power, hinder the identification of actual treatment effects, and increase the likelihood of false negatives (Type II errors). This may obstruct the reliability and reproducibility of research results.
The administration of sildenafil demonstrated promising effects in mitigating non-reassuring FHR patterns, particularly tachycardia and bradycardia, and contributed to a reduction in the rate of cesarean deliveries. Despite these benefits, neonatal outcomes remained comparable to those observed in the control group. These findings suggest potential relevance for future clinical investigations of sildenafil citrate in human populations. However, further studies involving larger cohorts are warranted to strengthen the evidence and allow for more definitive conclusions. In designing subsequent trials, the aforementioned limitations should be carefully considered.
Conclusion
Sildenafil improves non-reassuring FHR patterns, especially tachycardia and bradycardia, and decreases the rate of cesarean deliveries.
Acknowledgments: We acknowledge Yasuj University of Medical Sciences for providing research funding.
Ethical Permissions: The ethical guidelines for research and the required approvals were obtained from the vice-chancellor of research at Yasuj University of Medical Sciences (IR.YUMS.REC.1402.037).
Conflicts of Interests: The authors declared that they have no competing interests.
Authors' Contribution: Asadikalameh Z (First Author), Methodologist/Main Researcher/Discussion Writer (40%); Golshahi Chamandani F (Second Author), Introduction Writer/Assistant Researcher (20%); Sharifi M (Third Author), Methodologist/Assistant Researcher/Discussion Writer/Statistical Analyst (40%)
Funding/Support: The research received funding from Yasuj University of Medical Sciences.
Keywords:
References
1. Lear CA, Westgate JA, Ugwumadu A, Nijhuis JG, Stone PR, Georgieva A, et al. Understanding fetal heart rate patterns that may predict antenatal and intrapartum neural injury. Semin Pediatr Neurol. 2018;28:3-16. [Link] [DOI:10.1016/j.spen.2018.05.002]
2. Vintzileos AM, Smulian JC. Abnormal fetal heart rate patterns caused by pathophysiologic processes other than fetal acidemia. Am J Obstet Gynecol. 2023;228(5S):S1144-57. [Link] [DOI:10.1016/j.ajog.2022.05.002]
3. Lear CA, Ugwumadu A, Bennet L, Gunn AJ. An update of our understanding of fetal heart rate patterns in health and disease. Semin Pediatr Neurol. 2023;47:101072. [Link] [DOI:10.1016/j.spen.2023.101072]
4. Roozbeh N, Montazeri F, Farashah MV, Mehrnoush V, Darsareh F. Proposing a machine learning-based model for predicting nonreassuring fetal heart. Sci Rep. 2025;15(1):7812. [Link] [DOI:10.1038/s41598-025-92810-2]
5. Nageotte MP. Fetal heart rate monitoring. Semin Fetal Neonatal Med. 2015;20(3):144-8. [Link] [DOI:10.1016/j.siny.2015.02.002]
6. Cartledge J, Eardley I. Sildenafil. Expert Opin Pharmacother. 1999;1(1):137-47. [Link] [DOI:10.1517/14656566.1.1.137]
7. Ghofrani HA, Osterloh IH, Grimminger F. Sildenafil: From angina to erectile dysfunction to pulmonary hypertension and beyond. Nat Rev Drug Discov. 2006;5(8):689-702. [Link] [DOI:10.1038/nrd2030]
8. Ahmed WS, Geethakumari AM, Biswas KH. Phosphodiesterase 5 (PDE5): Structure-function regulation and therapeutic applications of inhibitors. Biomed Pharmacother. 2021;134:111128. [Link] [DOI:10.1016/j.biopha.2020.111128]
9. Kass DA, Champion HC, Beavo JA. Phosphodiesterase type 5: Expanding roles in cardiovascular regulation. Circ Res. 2007;101(11):1084-95. [Link] [DOI:10.1161/CIRCRESAHA.107.162511]
10. Dilworth MR, Andersson I, Renshall LJ, Cowley E, Baker P, Greenwood S, et al. Sildenafil citrate increases fetal weight in a mouse model of fetal growth restriction with a normal vascular phenotype. PLoS One. 2013;8(10):e77748. [Link] [DOI:10.1371/journal.pone.0077748]
11. Oyston C, Stanley JL, Oliver MH, Bloomfield FH, Baker PN. Maternal administration of sildenafil citrate alters fetal and placental growth and fetal-placental vascular resistance in the growth-restricted ovine fetus. Hypertension. 2016;68(3):760-7. [Link] [DOI:10.1161/HYPERTENSIONAHA.116.07662]
12. Samangaya RA, Mires G, Shennan A, Skillern L, Howe D, McLeod A, et al. A randomised, double-blinded, placebo-controlled study of the phosphodiesterase type 5 inhibitor sildenafil for the treatment of preeclampsia. Hypertens Pregnancy. 2009;28(4):369-82. [Link] [DOI:10.3109/10641950802601278]
13. Trapani A, Gonçalves LF, Trapani TF, Vieira S, Pires M, Pires MMS. Perinatal and hemodynamic evaluation of sildenafil citrate for preeclampsia treatment: A randomized controlled trial. Obstet Gynecol. 2016;128(2):253-9. [Link] [DOI:10.1097/AOG.0000000000001518]
14. Mohammadi E, Noei Teymoordash S, Norouzi AR, Norouzi F, Norouzi HR. Comparison of the effect of nifedipine alone and the combination of nifedipine and sildenafil in delaying preterm labor: A randomized clinical trial. J Family Reprod Health. 2021;15(2):112-7. [Link] [DOI:10.18502/jfrh.v15i2.6452]
15. Turner J, Dunn L, Tarnow-Mordi W, Flatley C, Flenady V, Kumar S. Safety and efficacy of sildenafil citrate to reduce operative birth for intrapartum fetal compromise at term: A phase 2 randomized controlled trial. Am J Obstet Gynecol. 2020;222(5):401-14. [Link] [DOI:10.1016/j.ajog.2020.01.025]
16. Cuschieri S. The CONSORT statement. Saudi J Anaesth. 2019;13(Suppl 1):S27-30. [Link] [DOI:10.4103/sja.SJA_559_18]
17. The American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 106: Intrapartum fetal heart rate monitoring: Nomenclature, interpretation, and general management principles. Obstet Gynecol. 2009;114(1):192-202. [Link] [DOI:10.1097/AOG.0b013e3181aef106]
18. Villanueva-García D, Mota-Rojas D, Hernández-González R, Sánchez-Aparicio P, Alonso-Spilsbury M, Trujillo-Ortega ME, et al. A systematic review of experimental and clinical studies of sildenafil citrate for intrauterine growth restriction and pre-term labour. J Obstet Gynaecol. 2007;27(3):255-9. [Link] [DOI:10.1080/01443610701194978]
19. Reynolds LP, Caton JS, Redmer DA, Grazul-Bilska AT, Vonnahme KA, Borowicz PP, et al. Evidence for altered placental blood flow and vascularity in compromised pregnancies. J Physiol. 2006;572(Pt 1):51-8. [Link] [DOI:10.1113/jphysiol.2005.104430]
20. Redmer DA, Wallace JM, Reynolds LP. Effect of nutrient intake during pregnancy on fetal and placental growth and vascular development. Domest Anim Endocrinol. 2004;27(3):199-217. [Link] [DOI:10.1016/j.domaniend.2004.06.006]
21. Reynolds LP, Borowicz PP, Caton JS, Vonnahme KA, Luther JS, Buchanan DS, et al. Uteroplacental vascular development and placental function: An update. Int J Dev Biol. 2010;54(2-3):355-66. [Link] [DOI:10.1387/ijdb.082799lr]
22. Rahimi F, Safavi Ardabili N, Asgharpoor H, Darsareh F. Effects of single-course betamethasone on the outcomes of late preterm neonates. Cureus. 2023;15(10):e46672. [Link] [DOI:10.7759/cureus.46672]
23. Darsareh F, Ranjbar A, Farashah MV, Mehrnoush V, Shekari M, Jahromi MS. Application of machine learning to identify risk factors of birth asphyxia. BMC Pregnancy Childbirth. 2023;23(1):156. [Link] [DOI:10.1186/s12884-023-05486-9]
24. Shekari M, Jahromi MS, Ranjbar A, Mehrnoush V, Darsareh F, Roozbeh N. The incidence and risk factors of meconium amniotic fluid in singleton pregnancies: an experience of a tertiary hospital in Iran. BMC Pregnancy Childbirth. 2022;22(1):930. [Link] [DOI:10.1186/s12884-022-05285-8]
25. Ranjbar A, Mehrnoush V, Darsareh F, Pariafsay F, Shirzadfardjahromi M, Shekari M. The incidence and outcomes of late-term pregnancy. Cureus. 2023;15(1):e33550. [Link] [DOI:10.7759/cureus.33550]
26. Rezaei Ghamsari S, Taeidi E, Darsareh F, Mehrnoush V. Analysis of cesarean section rates in a public tertiary hospital during teaching and non-teaching periods using the Robson ten group classification system. Cureus. 2023;15(8):e43838. [Link] [DOI:10.7759/cureus.43838]
27. Kumar S, Tarnow-Mordi W, Mol BW, Flenady V, Liley HG, Badawi N, et al. Intrapartum sildenafil to improve perinatal outcomes: A randomized clinical trial. JAMA. 2025;334(2):149-59. [Link] [DOI:10.1001/jama.2025.7710]
28. Dunn L, Greer R, Flenady V, Kumar S. Sildenafil in pregnancy: A systematic review of maternal tolerance and obstetric and perinatal outcomes. Fetal Diagn Ther. 2017;41(2):81-8. [Link] [DOI:10.1159/000453062]
29. Mehrnoush V, Ranjbar A, Banihashemi F, Darsareh F, Shekari M, Shirzadfardjahromi M. Urban-rural differences in the pregnancy-related adverse outcome. Gynecol Obstet Clin Med. 2023;3(1):51-5. [Link] [DOI:10.1016/j.gocm.2022.12.001]
30. Darsareh F, Aghamolaei T, Rajaei M, Madani A. Determinants of caesarean birth on maternal demand in the Islamic Republic of Iran: A review. East Mediterr Health J. 2017;23(6):441-8. [Link] [DOI:10.26719/2017.23.6.441]










