Volume 5, Issue 4 (2024)
J Clinic Care Skill 2024, 5(4): 189-195 |
Back to browse issues page
Article Type:
Subject:
Ethics code: IR.YUMS.REC.1401.132
History
Received: 2024/10/11 | Accepted: 2024/11/28 | Published: 2024/12/2
Received: 2024/10/11 | Accepted: 2024/11/28 | Published: 2024/12/2
How to cite this article
Jahantab M, Mohsenpour A, Salaminia S, Mehrabi Sisakht S, Roustaei N, Malekzadeh J et al . Effect of Carbon Dioxide Intraperitoneal Insufflation Pressure on Coagulation Status and Laboratory Liver Function Test After Laparoscopic Cholecystectomy. J Clinic Care Skill 2024; 5 (4) :189-195
URL: http://jccs.yums.ac.ir/article-1-299-en.html
URL: http://jccs.yums.ac.ir/article-1-299-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
M.B. Jahantab1 
 , A. Mohsenpour1
, A. Mohsenpour1 
 , Sh. Salaminia *1
, Sh. Salaminia *1 
 , S. Mehrabi Sisakht1
, S. Mehrabi Sisakht1 
 , N. Roustaei2
, N. Roustaei2 
 , J.M. Malekzadeh3
, J.M. Malekzadeh3 
 , M.J. Yavari Barhaghtalab1
, M.J. Yavari Barhaghtalab1 


 , A. Mohsenpour1
, A. Mohsenpour1 
 , Sh. Salaminia *1
, Sh. Salaminia *1 
 , S. Mehrabi Sisakht1
, S. Mehrabi Sisakht1 
 , N. Roustaei2
, N. Roustaei2 
 , J.M. Malekzadeh3
, J.M. Malekzadeh3 
 , M.J. Yavari Barhaghtalab1
, M.J. Yavari Barhaghtalab1 

1- “Department of Surgery, School of Medicine” and “Shahid Beheshti Hospital”, Yasuj University of Medical Sciences, Yasuj, Iran
2- “Department of Biostatistics and Epidemiology, School of Health” and “Social Determinants of Health Research Center”, Yasuj University of Medical Sciences, Yasuj, Iran
3- Department of Nutrition, School of Health, Yasuj University of Medical Sciences, Yasuj, Iran
2- “Department of Biostatistics and Epidemiology, School of Health” and “Social Determinants of Health Research Center”, Yasuj University of Medical Sciences, Yasuj, Iran
3- Department of Nutrition, School of Health, Yasuj University of Medical Sciences, Yasuj, Iran
Full-Text (HTML) (747 Views)
Introduction
Gallstone disease is the manifestation of stones in the gallbladder or common bile duct. Chronic abnormal gallbladder motility and emptying may cause gallbladder stones leading to chronic cholelithiasis. Manifestations of the gallstone disease might be mild and non-specific or severe and painful. In the case of acute biliary colic recurrent pain attacks occur, and while the pain continues for more than a day, surgical intervention is inevitable [1, 2].
Approximately 700,000 people require gallbladder surgery every year, and 80-90% of them are candidates for laparoscopic cholecystectomy. Recent studies have shown that every year more than 500,000 Americans undergo laparoscopic cholecystectomy [3].
The adoption of laparoscopic cholecystectomy in the treatment of gallbladder diseases created a new range of intraoperative and postoperative complications. Minor complications (biliary and non-biliary) are usually treated conservatively. Major complications (biliary and vascular) are life-threatening and increase the mortality rate, so for their treatment, there is a need to convert to open surgery. The most serious complications associated with a high mortality rate include damage to the common bile duct with an incidence of 0.1-0.6%, and damage to large blood vessels 0.4-1.22%. The most common complication is iatrogenic perforation of the gallbladder with a shed gallstone with an incidence of 10-30% [4-6]. Considering the side effects of blowing CO2 gas and the mechanical effects of intraperitoneal CO2 insufflation and increased intraabdominal pressure (IAP), the veins may be compressed and cause hemodynamic consequences [7, 8].
A significant postoperative decrease in activated partial thromboplastin time (aPTT: Activated partial thromboplastin time) and antithrombin III indicates the activation of coagulation, while the increase in d-dimer indicates the activation of fibrinolysis. Age, body mass index and duration of CO2 intraperitoneal insufflation are associated with significant activation of coagulation and fibrinolysis. CO2 intraperitoneal insufflation increases coagulation activation and fibrinolysis associated with laparoscopic cholecystectomy. Patients with risk factors such as old age, obesity, or with a long-expected duration of laparoscopic surgery are likely to have significant coagulation activation, making them a high-risk group for postoperative deep vein thrombosis [8, 9].
Laparoscopic cholecystectomy disturbs liver function tests (LFTs) in many patients. Observation of postoperative changes in the level of LFTs after open cholecystectomy has been reported in various studies. Changes in postoperative LFTs reflect a hemodynamic disturbance in hepatic and abdominal visceral blood flow, anesthetic hepatotoxicity, and biliary injuries [10-14]. The sensitivity of LFTs in detecting bile obstruction is more than 90%. Aspartate aminotransferase (AST), and alanine transaminase (ALT) are generally considered a measure of liver cell function. Alkaline phosphatase (ALP) levels increase during obstruction of the bile duct system. Bilirubin levels can be elevated due to hemolysis or obstruction of bile flow. Very high levels of serum transaminases can also indicate common bile duct (CBD) stones [13, 14].
In the study by Aggarwal et al. high-pressure pneumoperitoneum (>14 mm of Hg) has increased significantly serum Aspartate Aminotransferase (AST) and Alkaline phosphatase (ALP) compare to low-pressure of pneumoperitoneum (<10 mm of Hg). However, in the study by Zagorac et al., there has been not significant difference between low-pressure pneumoperitoneum (12 mm Hg; N=78) and high-pressure pneumoperitoneum (14 mmHg) in bilirubin, AST, GGT, LDH, albumin, and fibrinogen between [15]. However, ALT changes significantly on the 30th postoperative day, and there is a statistical difference for all hemato-parameters, independent of the level of pneumoperitoneum pressure [16]. Therefore, given the conflicting results of studies on the complications of laparoscopic cholecystectomy after CO2 intraperitoneal insufflation, the present study was conducted with the aim of comparing the effect of low-pressure CO2 intraperitoneal insufflation (12-15 mmHg), and high-pressure CO2 intraperitoneal insufflation (16-20 mmHg) on the patient's coagulation status and liver function test after laparoscopic cholecystectomy.
Materials and Methods
Design
The present study was a randomized clinical trial that was registered in the Iranian Registry of Clinical Trial with ID number: IRCT20220421054608N1. The study was conducted at Shahid Beheshti and Shahid Jalil Hospitals affiliated with Yasuj University of Medical Sciences, Yasuj, Iran.
Participants and sampling
In this randomized clinical trial, the study population included the patients who were candidates for laparoscopic cholecystectomy in Shahid Beheshti and Shahid Jalil Hospitals affiliated with Yasuj University of Medical Sciences from 2022/12/20 to 2023/12/23.
Based on σ1=14.4, σ2=30.1, μ1=36.8, μ2=44, α=0.05, β=0.2, 1-β=0.80, z1-α/2=1.96, and below statistical formula, the sample size was estimated 134 patients, that considering a 20% dropout rate, the sample size increased to 150 patients (each group 75 patients) [17].
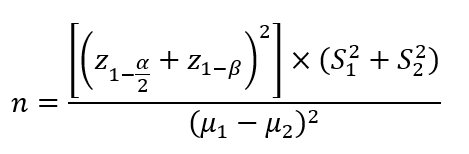
They were recruited using the convenience sampling method and allocated to two groups including two different CO2 insufflation pressure levels, either 12-15 or 16-20 mmHg by random sampling method. Since patients undergo surgery on a weekly basis, the first patient each week is chosen randomly. The patient will be considered as the group with CO2 pressure between 12-15 mmHg and the next patient as the group with CO2 pressure 16-20 mmHg.
150 patients who were candidates for laparoscopic cholecystectomy entered the study.
The inclusion criteria of the study were: candidates for elective laparoscopic cholecystectomy, patient consent to participate in the study, age range 18-70 years, absence of blood coagulation problems, absence of pregnancy, absence of previous abdominal surgery, absence of heart problems, and not taking anticoagulant drugs.
The exclusion criteria of the study were: the occurrence of any complications during the operation including bleeding, intra-abdominal pressure drop, conversion to open, the patient’s dissatisfaction, and noninformative or uncooperative patients.
Instruments, and data gathering methods
For evaluating the coagulation profile status, five milliliters (ml) of citrated blood (4.5 ml of blood+0.5 ml of citrate) were used. The requested coagulation profile tests were PTT, PT, and INR. The normal range of PT is 10.8-13.3 seconds and PTT is 31.4-48 seconds [18]. The AST and ALT concentrations were measured with a Hitachi 7600 automatic analyzer (Hitachi, Tokyo, Japan).
Intervention
In the present study, the intervention was using carbon dioxide intraperitoneal insufflation pressure in low-pressure (12-15 mmHg) and high-pressure (16-20 mmHg) in patients with laparoscopic cholecystectomy.
Laparoscopic cholecystectomies were performed successfully under general anesthesia. A standardized anesthesia protocol was used for all patients. Propofol 2-2.5 mg/kg and fentanyl 1 µg/kg were used for the induction of anesthesia. Muscle relaxation for endotracheal intubation was obtained with rocuronium 0.6 mg/kg. Patients were ventilated with volume-controlled ventilation (VCV) mode using an anesthesia device (Dräger Primus®; Dräger Medical Systems, Inc. Danvers, MA, USA). Tidal volume (Vt) was set as 6-8 ml per kg of ideal body weight and positive end-expiratory pressure (PEEP) was set as 5 cmH2O. The respiratory rate was adjusted to maintain normocarbia (PETCO2=32-36 mmHg). Maintenance of anesthesia was provided with sevoflurane (1.5-2%) with an oxygen-air mixture (FiO2=0.4). After the termination of the anesthesia procedure patients were placed in the Trendelenburg position, and the operation started with small infra umbilical incisions and the insertion of a Veress needle for CO2 intraperitoneal insufflation. Group 1 received low-pressure pneumoperitoneum (12-15 mmHg, 75 patients) and group 2 received high-pressure pneumoperitoneum (16-20 mmHg, 75 patients). Then, the patient was placed in the reverse Trendelenburg position with a slight left angle. All of the patients were operated on using a standard 4-port technique.
None of the patients received prophylactic Low Molecular Weight Heparin (LMWH). Elastic socks were used on each patient prior to surgery to prevent deep venous thrombosis.
Study outcomes
Research outcomes included coagulation factors profiles such as (prothrombin time (PT), partial thromboplastin time (PTT), International normalized ratio (INR), and bleeding time (BT), and liver function tests such as AST, ALT, ALP, total bilirubin (T.bili), and direct bilirubin (D.bili). The target tests of coagulation profile and liver function were measured and recorded the night before the operation and 24 hours after the operation.
Blinding
Neither the patient nor the analyst was aware of how the collected data was divided (double-blind).
Ethical considerations
The research followed the tenets of the Declaration of Helsinki and was started after approval by the independent research ethics committee (the ethics committee of the Yasuj University of Medical Sciences (IR.YUMS.REC.1401.132)), and was completed based on and according to a plan (proposal). For the study participants, an informed consent form was completed. The confidentiality and privacy of the patients were respected. The information of the participants was coded from the beginning and only the researcher had access to the patient's profile. Patients were not deprived of usual treatment protocols and no additional cost was imposed on the patients and insurance organizations.
Statistical analysis
Descriptive results were presented as mean±standard deviation (SD), median and interquartile difference or percentage considering the type of data. An independent or paired t-test was used to compare the two means in parameters with normal distribution, and in the case of non-normal data distribution, the Wilcoxon sign rank or Mann-Whitney test was applied. A p-value less than 0.05 was considered statistically significant. All data were analyzed using the SPSS software version 16.
Findings
150 patients participated in this research (75 patients in each group), however, only in the low-pressure CO2 group one patient drop out due to death. Therefore, data from about 74 patients in the low-pressure group, and 75 patients in the high-pressure group were analyzed. There was no significant difference between intervention and control groups in age, sex, and weight. The mean age in the group with less than 15 was 48.55±13.56 years, while in the group with more than 15, it was 49.34±12.49 years, with a p-value of 0.675, indicating no significant difference. The mean weight was 78.99±9.78 kg in the group with less than 15 and 76.26±10.41 kg in the group with more than 15, with a p-value of 0.097, also showing no significant difference. Regarding sex distribution, the group with less than 15 had 30 females (40%) and 45 males (60%), while the group with more than 15 had 43 females (56.6%) and 33 males (43.4%), with a p-value of 0.051, suggesting a trend toward significance.
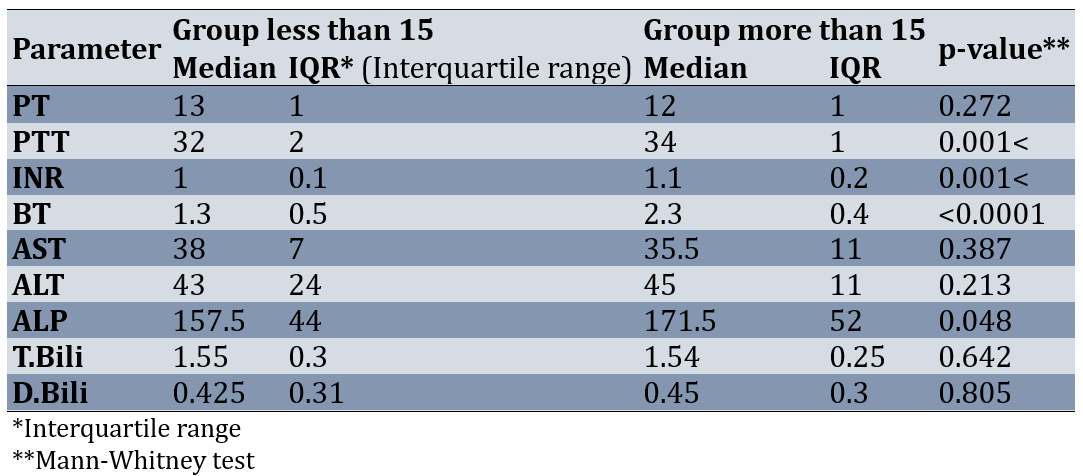
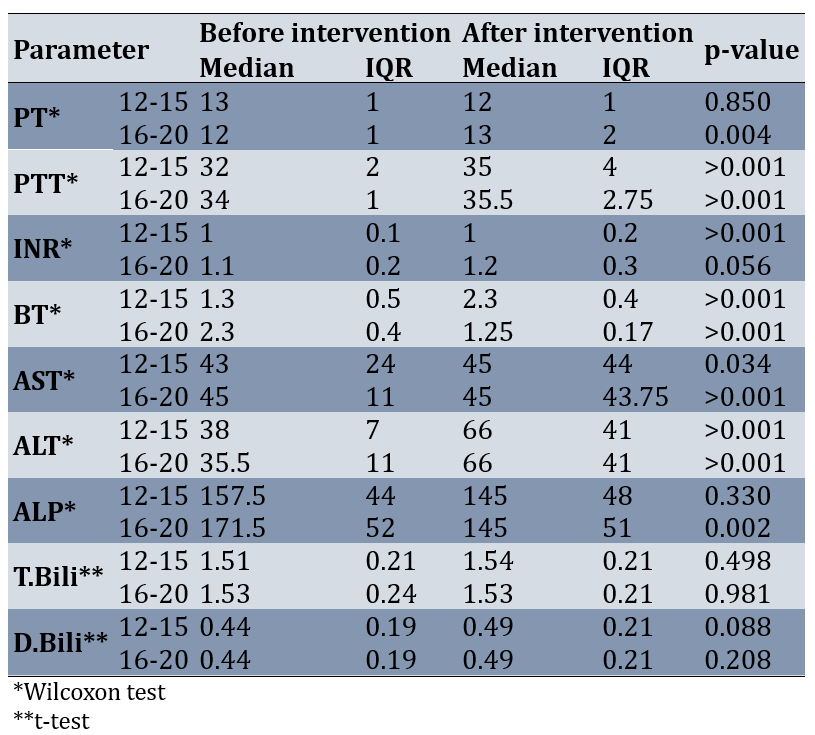
Before the intervention, the normality of the parameters of PT, PTT, INR, BT, AST, ALT, ALP, T.Bili, and D.Bili was checked in the separated two groups. The results of the Shapiro-Wilk test showed that at least for one of the groups, the distribution of PT, PTT, INR, BT, AST, ALT, ALP, T.Bili, and D.Bili was not normal before the intervention (p-value<0.05). Therefore, the Mann-Whitney test was used to compare the two groups. Results of comparing the two groups showed that some of the parameters such as PT, AST, ALT, T.Bili, and D.Bili had no statistically significant difference between the two groups before the intervention (less than 15 and more than 15; p-value>0.05). However, PTT (p-value=0.001), INR (p-value=0.001), BT (p-value=0.0001), and ALP (p-value=0.048) in the group more than 15 was significantly more than the group less than 15 (Table 1).
Table 1. Comparing the two groups according to some parameters before the operation
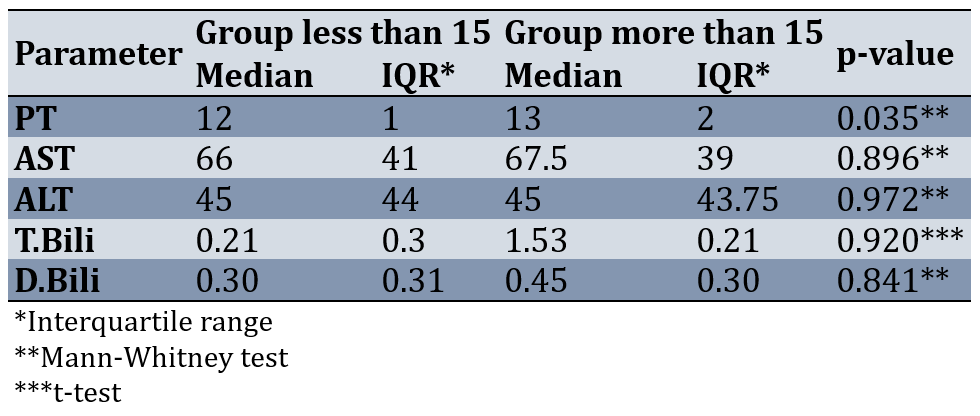
Results of comparing the two groups after the intervention showed that the distribution of PT between the two groups of less than 15 and more than 15 had a statistically significant difference (p-value=0.035). After the intervention, two groups were not significantly different in terms of other parameters, including AST, ALT, D.Bili, and T.Bili (p-value>0.05; Table 2).
Table 2. Results of comparing PT, AST, ALT, D.Bili, and T.Bili between the two groups after the intervention
Before the intervention, two groups were not significantly different in terms of he parameters of BT, PTT, INR, and ALP. So first, the differences (changes) before and after the intervention were calculated for these parameters, and then this difference before and after the intervention was compared in two groups.
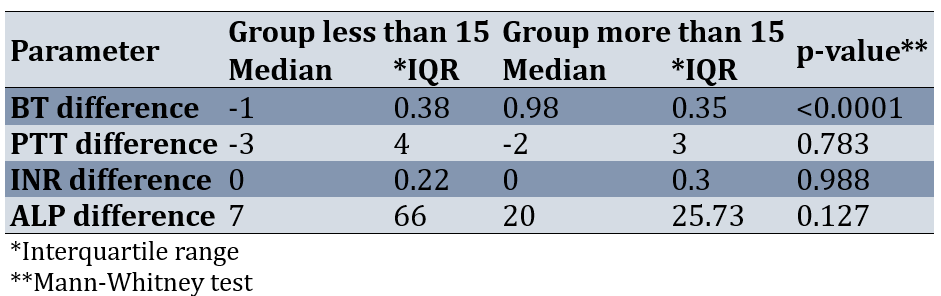
Results of comparing the two groups according to the difference before and after the operation showed that the changes of the BT parameter between the two groups of less than 15 and more than 15 before and after the intervention were statistically significant (p-value=0.0001). The changes before and after the intervention of INR, ALP, and PTT parameters between the two groups had no statistically significant difference (p-value>0.05; Table 3).
Table 3. Results of comparing the two groups according to the difference before and after the operation of BT, PTT, INR, and ALP
In the low-pressure group, PT, ALP, T.Bili, and D.Bili parameters were significantly different before and after the intervention (p-value>0.05). But PTT, INR, BT, ALT, and AST parameters were significantly higher after the intervention than before the intervention (p-value<0.05).
In the high-pressure group, INR, T.Bili, and D.Bili were not significantly different before and after the intervention (p-value>0.05). After the intervention, ALT, PT, PTT, and AST parameters were significantly higher than before the intervention (p-value<0.05). BT and ALP after the intervention were significantly lower than before the intervention (p-value<0.05; Table 4).
Table 4. Intragroup comparison of the investigated parameters before and after the operation in each group (less than 15 and more than 15)
Discussion
The present study was conducted with the aim of comparing the effect of low-pressure CO2 intraperitoneal insufflation (12-15 mmHg), and high-pressure CO2 intraperitoneal insufflation (16-20 mmHg) on the patient's coagulation status and liver function test after laparoscopic cholecystectomy. Results of the present study showed that, after laparoscopic cholecystectomy, in a group with high-pressure carbon dioxide (16-20 mmHg) compared to low-pressure (12-15 mmHg) intraperitoneal insufflation, indexes of PT increased and BT decreased significantly. However, there was no significant change in AST, ALT, D.Bili, T.Bili, PTT, INR, and ALP between groups after intervention.
In the low-pressure CO2 group (12-15 mmHg), PTT, INR, BT, ALT, and AST parameters were significantly higher after than before the intervention. Also, in the group of high-pressure CO2 (16-20 mmHg), PTT, ALT, and AST parameters after the intervention were significantly higher, and BT and ALP after the intervention were significantly lower .
The intraperitoneal insufflation of CO2 for laparoscopic cholecystectomy may lead to postoperative hypercoagulation (reduced coagulation activity and increased fibrinolytic activity) and LFT change in the patients, and thereby may increase the risks for the development of postoperative thrombosis and liver dysfunction; Patients may have risks for occurrence of thrombosis within 8 hours after the operation, to which attention should be paid in favor of preventing thrombosis and liver dysfunction [8, 19, 20] and this may be attributed to surgical trauma and pneumoperitoneum effects on the portal vein flow [21]. However, in the present study, PTT increased after the intervention in two groups, moreover, BT, INR, ALT, and AST increased in the group with low-pressure (12-15 mmHg) intraperitoneal insufflation, and ALP and BT decreased and PT, ALT, and AST increased in the group with high-pressure (16-20 mmHg) after the intervention. Therefore, intraperitoneal insufflation of CO2 for laparoscopic cholecystectomy may lead to changes in some coagulation factors and liver function tests.
According to the side effects of blowing CO2 gas and the mechanical effects of increased intraperitoneal insufflation pressure and intra-abdominal pressure (IAP), the veins may be compressed and cause an initial increase followed by a sustained decrease in cardiac preload. Cardiac vascular resistance (SVR: Cardiac Vascular Resistance) may be significantly reduced, and the magnitude of this effect is proportional to IAP [7]. In a study by Dexter et al., in healthy subjects who has undergone laparoscopic cholecystectomy, using transesophageal Doppler, he has found out that cardiac output decreased by up to 28% at a gas pressure of 15 mmHg, but the cardiac output was maintained at a gas pressure of 7 mmHg [22]. In an animal model, Iszaki et al. report that the IAP threshold that had the least effect on hemodynamic performance was less than 12 mmHg and recommended this pressure limit to avoid cardiovascular risks during CO2 insufflation [23].
The adverse effects of persistently elevated IAP on hepatic circulation are well documented. Hepatic blood flow varies in relation to IAP. Jakimowicz et al. investigate the effect of increased IAP on portal vein flow using duplex Doppler ultrasound in patients who underwent laparoscopic cholecystectomy. Portal blood flow is decreased by 37% at an IAP of 7 mmHg and by 53% when the IAP reaches 14 mmHg [24]. Also, the way the patient is positioned affects the changes in hepatic blood flow. Reverse Trendelenburg position is associated with a decrease in total hepatic, hepatic arterial, and portal venous blood flow [7].
In some studies, it was proved that laparoscopy has no effect on liver enzymes [12, 25]. However, positioning of the patient during Trendelenburg laparoscopy and increasing the intra-abdominal pressure due to CO2 gas administration and its absorption can also be effective on the liver [26]. Also, in the present study, some liver function tests in two groups increased. In a study by Donmez et al., intraperitoneal insufflation with 10 mmHg and 14 mmHg pressures has a significant increase in coagulation factors in both groups. According to increases in coagulation parameters, higher CO2 intraperitoneal insufflation pressure (14 mmHg) has a more negative effect on the coagulation cascade. In comparison to the results of this study, they suggest a lower intraperitoneal insufflation pressure (10 mmHg) during laparoscopic cholecystectomy because of the less pronounced effects on the coagulation status [27]. However, in the present study, PT, in high-pressure (16-20 mmHg) increased more than low-pressure intraperitoneal insufflation (12-15 mmHg), and the BT index Vice versa decreased. There was no significant difference between the two groups in INR and other indexes of coagulation and liver function tests between low- and high-pressure. In a study by Vecchio et al., postoperative PT values elevates slightly after laparoscopic surgery and caused activation of coagulation and fibrinolytic pathways. However, there is no significant change after the intervention compared to before [28]. The results indicated a significant increase in PTT of both groups, and PT in high-pressure CO2 after intervention, although the range of them was normal, and INR was in the normal range. The difference between the results of this study and other studies may be due to the fact that the laboratory tests were measured in different laboratories by different technicians with different normal ranges. In the present study, although in the low-pressure group, INR, ALT, and AST parameters, and in the high-pressure group, ALT, and AST parameters after intervention increased, however, liver function tests no change significantly between low- and high-pressure groups. In a study by Giraudo et al., the gasless technique causes smaller alterations in serological hepatic parameters than intraperitoneal insufflation pressure of 14 mmHg. Therefore, the use of low-pressure intraperitoneal insufflation is recommended for patients with severe hepatic failure [29]. In a study done by Hasukić, ALT after 24 and 48 hours and AST after 24 hours increases in the patients who underwent high-pressure laparoscopic cholecystectomy. The AST levels after 48 hours are statistically unchanged from the baseline in both groups. T.bili and ALP levels remain unchanged from baseline in both groups, without a significant difference between the two groups [30]. In the present study, ALT, and AST increased after intervention in both groups, but there was no difference between low- and high-pressure groups after 24 hours post-operation. In a study by Morino et al., postoperative increases in AST, ALT, bilirubin, and PT were seen. The increase of AST and ALT was statistically significant and correlated with both levels (10 versus 14 mmHg) and the duration of CO2 intraperitoneal insufflation. The duration and level of intraabdominal pressure are responsible for changes in hepatic function during laparoscopic procedures [31]. Maybe, the differences between the present and above studies were due to the duration of pressure and time of follow-up. In a study by Aggarwal et al. there was no significant difference in bilirubin and ALP in both groups after surgery, but AST and ALT increased significantly after laparoscopic cholecystectomy in patients in high-pressure CO2 (more than 14 mmHg) group compared to low-pressure CO2 (10-14 mmHg) [15]. However, in the present study, AST and ALT in low- and high-pressure groups increased significantly, but ALP decreased in the high-pressure group. Similar to the present study, Zagorac et al. in the study indicated that bilirubin, AST, and fibrinogen did not change significantly between low-, and high-pressure groups, but ALT changed between groups on the 30th surgery day [16].
Transient elevation of hepatic transaminases might be attributed to hepatocellular dysfunction secondary to CO2 intraperitoneal insufflation, diathermy, extruding liver, the branch of the hepatic artery injured, and general anesthesia [32].
The limitation of the study is that liver and coagulation tests were measured in different laboratories and had different normal ranges, and these were not taken into account. Another limitation is the short measurement time (24 hours) and it would have been better if laboratory tests had been measured 48 hours later.
It is suggested the similar study, that all laboratory tests measured by a laboratory, and by one technician. Moreover, the measurement repeat after 48 h.
Conclusion
PT elevation and BT reduction were observed with high-pressure CO2 compared to low-pressure CO2 intraperitoneal insufflation after laparoscopic cholecystectomy. Given the lack of significant difference in INR, and LFTs between the two groups and the normal range of PT and BT after laparoscopy, the level of intraperitoneal CO2 insufflation has no impact on coagulation and liver. Therefore, CO2 pressure of 12-20 can be used safely in laparoscopic cholecystectomy surgeries.
Although there was no significant change in liver function tests between the two groups after laparoscopic cholecystectomy, however some liver tests and coagulation factors changed in each group after intervention compared to before.
Acknowledgments: We all express our gratitude to the patients who kindly gave consent for participation in this thesis work and the publication of this manuscript. This paper is registered with research project number 980002 in the Vice-Chancellor for Research and Technology Development of Yasuj University of Medical Sciences, School of Medicine, Yasuj, Iran.
Ethical Permissions: This study is approved under the ethical code of IR.YUMS.REC.1401.132 by the ethics committee of Yasuj University of Medical Sciences, Yasuj, Iran. All methods were carried out under the ethical standards as laid down in the Declaration of Helsinki and its later amendments or comparable ethical standards. Written informed consent was obtained from the patients for participation.
Conflicts of Interests: The authors of this manuscript declare no competing interests.
Authors' Contribution: Jahantab MB (First Author), Introduction Writer/Original Researcher/Discussion Writer (15%); Mohsenpour A (Second Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer (14%); Salaminia Sh (Third Author), Introduction Writer/Original Researcher/Discussion Writer (15%); Mehrabi Sisakht S (Fourth Author), Introduction Writer/Methodologist/Assistant Researcher (14%); Roustaei N (Fifth Author), Methodologist/Assistant Researcher/Discussion Writer (14%); Malekzadeh JM (Sixth Author), Methodologist/Assistant Researcher/Discussion Writer (14%); Yavari Barhaghtalab MJ (Seventh Author), Assistant Researcher/Statistical Analyst (14%)
Funding/Support: Not received.
Gallstone disease is the manifestation of stones in the gallbladder or common bile duct. Chronic abnormal gallbladder motility and emptying may cause gallbladder stones leading to chronic cholelithiasis. Manifestations of the gallstone disease might be mild and non-specific or severe and painful. In the case of acute biliary colic recurrent pain attacks occur, and while the pain continues for more than a day, surgical intervention is inevitable [1, 2].
Approximately 700,000 people require gallbladder surgery every year, and 80-90% of them are candidates for laparoscopic cholecystectomy. Recent studies have shown that every year more than 500,000 Americans undergo laparoscopic cholecystectomy [3].
The adoption of laparoscopic cholecystectomy in the treatment of gallbladder diseases created a new range of intraoperative and postoperative complications. Minor complications (biliary and non-biliary) are usually treated conservatively. Major complications (biliary and vascular) are life-threatening and increase the mortality rate, so for their treatment, there is a need to convert to open surgery. The most serious complications associated with a high mortality rate include damage to the common bile duct with an incidence of 0.1-0.6%, and damage to large blood vessels 0.4-1.22%. The most common complication is iatrogenic perforation of the gallbladder with a shed gallstone with an incidence of 10-30% [4-6]. Considering the side effects of blowing CO2 gas and the mechanical effects of intraperitoneal CO2 insufflation and increased intraabdominal pressure (IAP), the veins may be compressed and cause hemodynamic consequences [7, 8].
A significant postoperative decrease in activated partial thromboplastin time (aPTT: Activated partial thromboplastin time) and antithrombin III indicates the activation of coagulation, while the increase in d-dimer indicates the activation of fibrinolysis. Age, body mass index and duration of CO2 intraperitoneal insufflation are associated with significant activation of coagulation and fibrinolysis. CO2 intraperitoneal insufflation increases coagulation activation and fibrinolysis associated with laparoscopic cholecystectomy. Patients with risk factors such as old age, obesity, or with a long-expected duration of laparoscopic surgery are likely to have significant coagulation activation, making them a high-risk group for postoperative deep vein thrombosis [8, 9].
Laparoscopic cholecystectomy disturbs liver function tests (LFTs) in many patients. Observation of postoperative changes in the level of LFTs after open cholecystectomy has been reported in various studies. Changes in postoperative LFTs reflect a hemodynamic disturbance in hepatic and abdominal visceral blood flow, anesthetic hepatotoxicity, and biliary injuries [10-14]. The sensitivity of LFTs in detecting bile obstruction is more than 90%. Aspartate aminotransferase (AST), and alanine transaminase (ALT) are generally considered a measure of liver cell function. Alkaline phosphatase (ALP) levels increase during obstruction of the bile duct system. Bilirubin levels can be elevated due to hemolysis or obstruction of bile flow. Very high levels of serum transaminases can also indicate common bile duct (CBD) stones [13, 14].
In the study by Aggarwal et al. high-pressure pneumoperitoneum (>14 mm of Hg) has increased significantly serum Aspartate Aminotransferase (AST) and Alkaline phosphatase (ALP) compare to low-pressure of pneumoperitoneum (<10 mm of Hg). However, in the study by Zagorac et al., there has been not significant difference between low-pressure pneumoperitoneum (12 mm Hg; N=78) and high-pressure pneumoperitoneum (14 mmHg) in bilirubin, AST, GGT, LDH, albumin, and fibrinogen between [15]. However, ALT changes significantly on the 30th postoperative day, and there is a statistical difference for all hemato-parameters, independent of the level of pneumoperitoneum pressure [16]. Therefore, given the conflicting results of studies on the complications of laparoscopic cholecystectomy after CO2 intraperitoneal insufflation, the present study was conducted with the aim of comparing the effect of low-pressure CO2 intraperitoneal insufflation (12-15 mmHg), and high-pressure CO2 intraperitoneal insufflation (16-20 mmHg) on the patient's coagulation status and liver function test after laparoscopic cholecystectomy.
Materials and Methods
Design
The present study was a randomized clinical trial that was registered in the Iranian Registry of Clinical Trial with ID number: IRCT20220421054608N1. The study was conducted at Shahid Beheshti and Shahid Jalil Hospitals affiliated with Yasuj University of Medical Sciences, Yasuj, Iran.
Participants and sampling
In this randomized clinical trial, the study population included the patients who were candidates for laparoscopic cholecystectomy in Shahid Beheshti and Shahid Jalil Hospitals affiliated with Yasuj University of Medical Sciences from 2022/12/20 to 2023/12/23.
Based on σ1=14.4, σ2=30.1, μ1=36.8, μ2=44, α=0.05, β=0.2, 1-β=0.80, z1-α/2=1.96, and below statistical formula, the sample size was estimated 134 patients, that considering a 20% dropout rate, the sample size increased to 150 patients (each group 75 patients) [17].
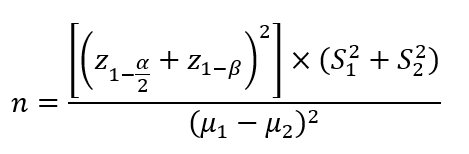
They were recruited using the convenience sampling method and allocated to two groups including two different CO2 insufflation pressure levels, either 12-15 or 16-20 mmHg by random sampling method. Since patients undergo surgery on a weekly basis, the first patient each week is chosen randomly. The patient will be considered as the group with CO2 pressure between 12-15 mmHg and the next patient as the group with CO2 pressure 16-20 mmHg.
150 patients who were candidates for laparoscopic cholecystectomy entered the study.
The inclusion criteria of the study were: candidates for elective laparoscopic cholecystectomy, patient consent to participate in the study, age range 18-70 years, absence of blood coagulation problems, absence of pregnancy, absence of previous abdominal surgery, absence of heart problems, and not taking anticoagulant drugs.
The exclusion criteria of the study were: the occurrence of any complications during the operation including bleeding, intra-abdominal pressure drop, conversion to open, the patient’s dissatisfaction, and noninformative or uncooperative patients.
Instruments, and data gathering methods
For evaluating the coagulation profile status, five milliliters (ml) of citrated blood (4.5 ml of blood+0.5 ml of citrate) were used. The requested coagulation profile tests were PTT, PT, and INR. The normal range of PT is 10.8-13.3 seconds and PTT is 31.4-48 seconds [18]. The AST and ALT concentrations were measured with a Hitachi 7600 automatic analyzer (Hitachi, Tokyo, Japan).
Intervention
In the present study, the intervention was using carbon dioxide intraperitoneal insufflation pressure in low-pressure (12-15 mmHg) and high-pressure (16-20 mmHg) in patients with laparoscopic cholecystectomy.
Laparoscopic cholecystectomies were performed successfully under general anesthesia. A standardized anesthesia protocol was used for all patients. Propofol 2-2.5 mg/kg and fentanyl 1 µg/kg were used for the induction of anesthesia. Muscle relaxation for endotracheal intubation was obtained with rocuronium 0.6 mg/kg. Patients were ventilated with volume-controlled ventilation (VCV) mode using an anesthesia device (Dräger Primus®; Dräger Medical Systems, Inc. Danvers, MA, USA). Tidal volume (Vt) was set as 6-8 ml per kg of ideal body weight and positive end-expiratory pressure (PEEP) was set as 5 cmH2O. The respiratory rate was adjusted to maintain normocarbia (PETCO2=32-36 mmHg). Maintenance of anesthesia was provided with sevoflurane (1.5-2%) with an oxygen-air mixture (FiO2=0.4). After the termination of the anesthesia procedure patients were placed in the Trendelenburg position, and the operation started with small infra umbilical incisions and the insertion of a Veress needle for CO2 intraperitoneal insufflation. Group 1 received low-pressure pneumoperitoneum (12-15 mmHg, 75 patients) and group 2 received high-pressure pneumoperitoneum (16-20 mmHg, 75 patients). Then, the patient was placed in the reverse Trendelenburg position with a slight left angle. All of the patients were operated on using a standard 4-port technique.
None of the patients received prophylactic Low Molecular Weight Heparin (LMWH). Elastic socks were used on each patient prior to surgery to prevent deep venous thrombosis.
Study outcomes
Research outcomes included coagulation factors profiles such as (prothrombin time (PT), partial thromboplastin time (PTT), International normalized ratio (INR), and bleeding time (BT), and liver function tests such as AST, ALT, ALP, total bilirubin (T.bili), and direct bilirubin (D.bili). The target tests of coagulation profile and liver function were measured and recorded the night before the operation and 24 hours after the operation.
Blinding
Neither the patient nor the analyst was aware of how the collected data was divided (double-blind).
Ethical considerations
The research followed the tenets of the Declaration of Helsinki and was started after approval by the independent research ethics committee (the ethics committee of the Yasuj University of Medical Sciences (IR.YUMS.REC.1401.132)), and was completed based on and according to a plan (proposal). For the study participants, an informed consent form was completed. The confidentiality and privacy of the patients were respected. The information of the participants was coded from the beginning and only the researcher had access to the patient's profile. Patients were not deprived of usual treatment protocols and no additional cost was imposed on the patients and insurance organizations.
Statistical analysis
Descriptive results were presented as mean±standard deviation (SD), median and interquartile difference or percentage considering the type of data. An independent or paired t-test was used to compare the two means in parameters with normal distribution, and in the case of non-normal data distribution, the Wilcoxon sign rank or Mann-Whitney test was applied. A p-value less than 0.05 was considered statistically significant. All data were analyzed using the SPSS software version 16.
Findings
150 patients participated in this research (75 patients in each group), however, only in the low-pressure CO2 group one patient drop out due to death. Therefore, data from about 74 patients in the low-pressure group, and 75 patients in the high-pressure group were analyzed. There was no significant difference between intervention and control groups in age, sex, and weight. The mean age in the group with less than 15 was 48.55±13.56 years, while in the group with more than 15, it was 49.34±12.49 years, with a p-value of 0.675, indicating no significant difference. The mean weight was 78.99±9.78 kg in the group with less than 15 and 76.26±10.41 kg in the group with more than 15, with a p-value of 0.097, also showing no significant difference. Regarding sex distribution, the group with less than 15 had 30 females (40%) and 45 males (60%), while the group with more than 15 had 43 females (56.6%) and 33 males (43.4%), with a p-value of 0.051, suggesting a trend toward significance.
Before the intervention, the normality of the parameters of PT, PTT, INR, BT, AST, ALT, ALP, T.Bili, and D.Bili was checked in the separated two groups. The results of the Shapiro-Wilk test showed that at least for one of the groups, the distribution of PT, PTT, INR, BT, AST, ALT, ALP, T.Bili, and D.Bili was not normal before the intervention (p-value<0.05). Therefore, the Mann-Whitney test was used to compare the two groups. Results of comparing the two groups showed that some of the parameters such as PT, AST, ALT, T.Bili, and D.Bili had no statistically significant difference between the two groups before the intervention (less than 15 and more than 15; p-value>0.05). However, PTT (p-value=0.001), INR (p-value=0.001), BT (p-value=0.0001), and ALP (p-value=0.048) in the group more than 15 was significantly more than the group less than 15 (Table 1).
Table 1. Comparing the two groups according to some parameters before the operation

Results of comparing the two groups after the intervention showed that the distribution of PT between the two groups of less than 15 and more than 15 had a statistically significant difference (p-value=0.035). After the intervention, two groups were not significantly different in terms of other parameters, including AST, ALT, D.Bili, and T.Bili (p-value>0.05; Table 2).
Table 2. Results of comparing PT, AST, ALT, D.Bili, and T.Bili between the two groups after the intervention

Before the intervention, two groups were not significantly different in terms of he parameters of BT, PTT, INR, and ALP. So first, the differences (changes) before and after the intervention were calculated for these parameters, and then this difference before and after the intervention was compared in two groups.
Results of comparing the two groups according to the difference before and after the operation showed that the changes of the BT parameter between the two groups of less than 15 and more than 15 before and after the intervention were statistically significant (p-value=0.0001). The changes before and after the intervention of INR, ALP, and PTT parameters between the two groups had no statistically significant difference (p-value>0.05; Table 3).
Table 3. Results of comparing the two groups according to the difference before and after the operation of BT, PTT, INR, and ALP

In the low-pressure group, PT, ALP, T.Bili, and D.Bili parameters were significantly different before and after the intervention (p-value>0.05). But PTT, INR, BT, ALT, and AST parameters were significantly higher after the intervention than before the intervention (p-value<0.05).
In the high-pressure group, INR, T.Bili, and D.Bili were not significantly different before and after the intervention (p-value>0.05). After the intervention, ALT, PT, PTT, and AST parameters were significantly higher than before the intervention (p-value<0.05). BT and ALP after the intervention were significantly lower than before the intervention (p-value<0.05; Table 4).
Table 4. Intragroup comparison of the investigated parameters before and after the operation in each group (less than 15 and more than 15)

Discussion
The present study was conducted with the aim of comparing the effect of low-pressure CO2 intraperitoneal insufflation (12-15 mmHg), and high-pressure CO2 intraperitoneal insufflation (16-20 mmHg) on the patient's coagulation status and liver function test after laparoscopic cholecystectomy. Results of the present study showed that, after laparoscopic cholecystectomy, in a group with high-pressure carbon dioxide (16-20 mmHg) compared to low-pressure (12-15 mmHg) intraperitoneal insufflation, indexes of PT increased and BT decreased significantly. However, there was no significant change in AST, ALT, D.Bili, T.Bili, PTT, INR, and ALP between groups after intervention.
In the low-pressure CO2 group (12-15 mmHg), PTT, INR, BT, ALT, and AST parameters were significantly higher after than before the intervention. Also, in the group of high-pressure CO2 (16-20 mmHg), PTT, ALT, and AST parameters after the intervention were significantly higher, and BT and ALP after the intervention were significantly lower .
The intraperitoneal insufflation of CO2 for laparoscopic cholecystectomy may lead to postoperative hypercoagulation (reduced coagulation activity and increased fibrinolytic activity) and LFT change in the patients, and thereby may increase the risks for the development of postoperative thrombosis and liver dysfunction; Patients may have risks for occurrence of thrombosis within 8 hours after the operation, to which attention should be paid in favor of preventing thrombosis and liver dysfunction [8, 19, 20] and this may be attributed to surgical trauma and pneumoperitoneum effects on the portal vein flow [21]. However, in the present study, PTT increased after the intervention in two groups, moreover, BT, INR, ALT, and AST increased in the group with low-pressure (12-15 mmHg) intraperitoneal insufflation, and ALP and BT decreased and PT, ALT, and AST increased in the group with high-pressure (16-20 mmHg) after the intervention. Therefore, intraperitoneal insufflation of CO2 for laparoscopic cholecystectomy may lead to changes in some coagulation factors and liver function tests.
According to the side effects of blowing CO2 gas and the mechanical effects of increased intraperitoneal insufflation pressure and intra-abdominal pressure (IAP), the veins may be compressed and cause an initial increase followed by a sustained decrease in cardiac preload. Cardiac vascular resistance (SVR: Cardiac Vascular Resistance) may be significantly reduced, and the magnitude of this effect is proportional to IAP [7]. In a study by Dexter et al., in healthy subjects who has undergone laparoscopic cholecystectomy, using transesophageal Doppler, he has found out that cardiac output decreased by up to 28% at a gas pressure of 15 mmHg, but the cardiac output was maintained at a gas pressure of 7 mmHg [22]. In an animal model, Iszaki et al. report that the IAP threshold that had the least effect on hemodynamic performance was less than 12 mmHg and recommended this pressure limit to avoid cardiovascular risks during CO2 insufflation [23].
The adverse effects of persistently elevated IAP on hepatic circulation are well documented. Hepatic blood flow varies in relation to IAP. Jakimowicz et al. investigate the effect of increased IAP on portal vein flow using duplex Doppler ultrasound in patients who underwent laparoscopic cholecystectomy. Portal blood flow is decreased by 37% at an IAP of 7 mmHg and by 53% when the IAP reaches 14 mmHg [24]. Also, the way the patient is positioned affects the changes in hepatic blood flow. Reverse Trendelenburg position is associated with a decrease in total hepatic, hepatic arterial, and portal venous blood flow [7].
In some studies, it was proved that laparoscopy has no effect on liver enzymes [12, 25]. However, positioning of the patient during Trendelenburg laparoscopy and increasing the intra-abdominal pressure due to CO2 gas administration and its absorption can also be effective on the liver [26]. Also, in the present study, some liver function tests in two groups increased. In a study by Donmez et al., intraperitoneal insufflation with 10 mmHg and 14 mmHg pressures has a significant increase in coagulation factors in both groups. According to increases in coagulation parameters, higher CO2 intraperitoneal insufflation pressure (14 mmHg) has a more negative effect on the coagulation cascade. In comparison to the results of this study, they suggest a lower intraperitoneal insufflation pressure (10 mmHg) during laparoscopic cholecystectomy because of the less pronounced effects on the coagulation status [27]. However, in the present study, PT, in high-pressure (16-20 mmHg) increased more than low-pressure intraperitoneal insufflation (12-15 mmHg), and the BT index Vice versa decreased. There was no significant difference between the two groups in INR and other indexes of coagulation and liver function tests between low- and high-pressure. In a study by Vecchio et al., postoperative PT values elevates slightly after laparoscopic surgery and caused activation of coagulation and fibrinolytic pathways. However, there is no significant change after the intervention compared to before [28]. The results indicated a significant increase in PTT of both groups, and PT in high-pressure CO2 after intervention, although the range of them was normal, and INR was in the normal range. The difference between the results of this study and other studies may be due to the fact that the laboratory tests were measured in different laboratories by different technicians with different normal ranges. In the present study, although in the low-pressure group, INR, ALT, and AST parameters, and in the high-pressure group, ALT, and AST parameters after intervention increased, however, liver function tests no change significantly between low- and high-pressure groups. In a study by Giraudo et al., the gasless technique causes smaller alterations in serological hepatic parameters than intraperitoneal insufflation pressure of 14 mmHg. Therefore, the use of low-pressure intraperitoneal insufflation is recommended for patients with severe hepatic failure [29]. In a study done by Hasukić, ALT after 24 and 48 hours and AST after 24 hours increases in the patients who underwent high-pressure laparoscopic cholecystectomy. The AST levels after 48 hours are statistically unchanged from the baseline in both groups. T.bili and ALP levels remain unchanged from baseline in both groups, without a significant difference between the two groups [30]. In the present study, ALT, and AST increased after intervention in both groups, but there was no difference between low- and high-pressure groups after 24 hours post-operation. In a study by Morino et al., postoperative increases in AST, ALT, bilirubin, and PT were seen. The increase of AST and ALT was statistically significant and correlated with both levels (10 versus 14 mmHg) and the duration of CO2 intraperitoneal insufflation. The duration and level of intraabdominal pressure are responsible for changes in hepatic function during laparoscopic procedures [31]. Maybe, the differences between the present and above studies were due to the duration of pressure and time of follow-up. In a study by Aggarwal et al. there was no significant difference in bilirubin and ALP in both groups after surgery, but AST and ALT increased significantly after laparoscopic cholecystectomy in patients in high-pressure CO2 (more than 14 mmHg) group compared to low-pressure CO2 (10-14 mmHg) [15]. However, in the present study, AST and ALT in low- and high-pressure groups increased significantly, but ALP decreased in the high-pressure group. Similar to the present study, Zagorac et al. in the study indicated that bilirubin, AST, and fibrinogen did not change significantly between low-, and high-pressure groups, but ALT changed between groups on the 30th surgery day [16].
Transient elevation of hepatic transaminases might be attributed to hepatocellular dysfunction secondary to CO2 intraperitoneal insufflation, diathermy, extruding liver, the branch of the hepatic artery injured, and general anesthesia [32].
The limitation of the study is that liver and coagulation tests were measured in different laboratories and had different normal ranges, and these were not taken into account. Another limitation is the short measurement time (24 hours) and it would have been better if laboratory tests had been measured 48 hours later.
It is suggested the similar study, that all laboratory tests measured by a laboratory, and by one technician. Moreover, the measurement repeat after 48 h.
Conclusion
PT elevation and BT reduction were observed with high-pressure CO2 compared to low-pressure CO2 intraperitoneal insufflation after laparoscopic cholecystectomy. Given the lack of significant difference in INR, and LFTs between the two groups and the normal range of PT and BT after laparoscopy, the level of intraperitoneal CO2 insufflation has no impact on coagulation and liver. Therefore, CO2 pressure of 12-20 can be used safely in laparoscopic cholecystectomy surgeries.
Although there was no significant change in liver function tests between the two groups after laparoscopic cholecystectomy, however some liver tests and coagulation factors changed in each group after intervention compared to before.
Acknowledgments: We all express our gratitude to the patients who kindly gave consent for participation in this thesis work and the publication of this manuscript. This paper is registered with research project number 980002 in the Vice-Chancellor for Research and Technology Development of Yasuj University of Medical Sciences, School of Medicine, Yasuj, Iran.
Ethical Permissions: This study is approved under the ethical code of IR.YUMS.REC.1401.132 by the ethics committee of Yasuj University of Medical Sciences, Yasuj, Iran. All methods were carried out under the ethical standards as laid down in the Declaration of Helsinki and its later amendments or comparable ethical standards. Written informed consent was obtained from the patients for participation.
Conflicts of Interests: The authors of this manuscript declare no competing interests.
Authors' Contribution: Jahantab MB (First Author), Introduction Writer/Original Researcher/Discussion Writer (15%); Mohsenpour A (Second Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer (14%); Salaminia Sh (Third Author), Introduction Writer/Original Researcher/Discussion Writer (15%); Mehrabi Sisakht S (Fourth Author), Introduction Writer/Methodologist/Assistant Researcher (14%); Roustaei N (Fifth Author), Methodologist/Assistant Researcher/Discussion Writer (14%); Malekzadeh JM (Sixth Author), Methodologist/Assistant Researcher/Discussion Writer (14%); Yavari Barhaghtalab MJ (Seventh Author), Assistant Researcher/Statistical Analyst (14%)
Funding/Support: Not received.
Keywords:
References
1. Jahantab MB, Salehi V, Mehrabi S, Abedini L, Yavari Barhaghtalab MJ. Cholecystomegaly: A case report and review of the literature. Case Rep Gastrointest Med. 2020;2020:8825167. [Link] [DOI:10.1155/2020/8825167]
2. Jahantab MB, Safaripour AA, Hassanzadeh S, Yavari Barhaghtalab MJ. Demographic, chemical, and helicobacter pylori positivity assessment in different types of gallstones and the bile in a random sample of cholecystectomied Iranian patients with cholelithiasis. Can J Gastroenterol Hepatol. 2021;2021:3351352. [Link] [DOI:10.1155/2021/3351352]
3. Chandra S, Friesen C, Attard TM. Trends in the epidemiology of pediatric acute and chronic cholecystitis-related admissions in the USA: A nationwide emergency department and inpatient sample study. J Investig Med. 2019;67(8):1155-9. [Link] [DOI:10.1136/jim-2018-000948]
4. McKinley SK, Brunt LM, Schwaitzberg SD. Prevention of bile duct injury: The case for incorporating educational theories of expertise. Surg Endosc. 2014;28(12):3385-91. [Link] [DOI:10.1007/s00464-014-3605-8]
5. Kaushik R. Bleeding complications in laparoscopic cholecystectomy: Incidence, mechanisms, prevention and management. J Minim Access Surg. 2010;6(3):59-65. [Link] [DOI:10.4103/0972-9941.68579]
6. Radunovic M, Lazovic R, Popovic N, Magdelinic M, Bulajic M, Radunovic L, et al. Complications of laparoscopic cholecystectomy: Our experience from a retrospective analysis. Open Access Maced J Med Sci. 2016;4(4):641-6. [Link] [DOI:10.3889/oamjms.2016.128]
7. Leonard IE, Cunningham AJ. Anaesthetic considerations for laparoscopic cholecystectomy. Best Pract Res Clin Anaesthesiol. 2002;16(1):1-20. [Link] [DOI:10.1053/bean.2001.0204]
8. Garg PK, Teckchandani N, Hadke NS, Chander J, Nigam S, Puri SK. Alteration in coagulation profile and incidence of DVT in laparoscopic cholecystectomy. Int J Surg. 2009;7(2):130-5. [Link] [DOI:10.1016/j.ijsu.2008.12.036]
9. Jahantab MB, Mehrabi S, Salehi V, Abedini L, Yavari Barhaghtalab MJ. Portal vein thrombosis following total colectomy due to colonic inertia: A case report and evaluation of risk factors. Case Rep Hematol. 2021;2021:8895206. [Link] [DOI:10.1155/2021/8895206]
10. Clarke RS, Doggart JR, Lavery T. Changes in liver function after different types of surgery. Br J Anaesth. 1976;48(2):119-28. [Link] [DOI:10.1093/bja/48.2.119]
11. Evans C, Evans M, Pollock AV. The incidence and causes of postoperative jaundice. A prospective study. Br J Anaesth. 1974;46(7):520-5. [Link] [DOI:10.1093/bja/46.7.520]
12. Halevy A, Gold-Deutch R, Negri M, Lin G, Shlamkovich N, Evans S, et al. Are elevated liver enzymes and bilirubin levels significant after laparoscopic cholecystectomy in the absence of bile duct injury?. Ann Surg. 1994;219(4):362-4. [Link] [DOI:10.1097/00000658-199404000-00006]
13. Tham TC, Collins JS, Watson RG, Ellis PK, McIlrath EM. Diagnosis of common bile duct stones by intravenous cholangiography: Prediction by ultrasound and liver function tests compared with endoscopic retrograde cholangiography. Gastrointest Endosc. 1996;44(2):158-63. [Link] [DOI:10.1016/S0016-5107(96)70133-5]
14. Ahmad NZ. Routine testing of liver function before and after elective laparoscopic cholecystectomy: Is it necessary?. J Soc Laparoendosc Surg. 2011;15(1):65-9. [Link] [DOI:10.4293/108680811X13022985131291]
15. Aggarwal M, Kumar A, Garg S, Pruthi A. Effect of low pressure versus high pressure pneumoperitoneum on liver functions in laparoscopic cholecystectomy. Int J Anat Radiol Surg. 2020;9(2):SO1-3. [Link]
16. Zagorac Z, Zivic R, Milanovic M, Vekic B, Dakovic B, Bukumiric Z, et al. Changes in liver function tests after laparoscopic cholecystectomy with low-and high-pressure pneumoperitoneum. Eur Surg. 2019;51(2):61-5. [Link] [DOI:10.1007/s10353-019-0568-y]
17. Ghazanfar A, Ghazanfar AA, Asghar A. Comparison of high versus low intra-peritoneal carbon dioxide pressure on abdominal pain, hemodynamic symptoms and liver function tests in patients undergoing laparoscopic cholecystectomy. J Rawalpindi Med Coll. 2019;23(2). [Link]
18. Pathepchotiwong K, Tantiniti P, Sutthithampanich C. Study of normal values in coagulation profile. J Med Assoc Thai. 2001;84(6):877-81. [Link]
19. Amin B, Zhang C, Yan W, Sun Z, Zhang Y, Du D, et al. Effects of pneumoperitoneum of laparoscopic cholecystectomy on the coagulation system of patients: A prospective observational study. Chin Med J. 2014;127(14):2599-604. [Link]
20. Marakis G, Pavlidis TE, Ballas K, Karvounaris D, Rafailidis S, Sakantamis AK. Changes in coagulation and fibrinolysis during laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A. 2006;16(6):582-6. [Link] [DOI:10.1089/lap.2006.16.582]
21. Ntourakis D, Sergentanis TN, Georgiopoulos I, Papadopoulou E, Liasis L, Kritikos E, et al. Subclinical activation of coagulation and fibrinolysis in laparoscopic cholecystectomy: Do risk factors exist?. Int J Surg. 2011;9(5):374-7. [Link] [DOI:10.1016/j.ijsu.2011.02.011]
22. Dexter SP, Vucevic M, Gibson J, McMahon MJ. Hemodynamic consequences of high- and low-pressure capnoperitoneum during laparoscopic cholecystectomy. Surg Endosc. 1999;13(4):376-81. [Link] [DOI:10.1007/s004649900993]
23. Iszaki Y, Bandai Y, Shimomura K, Abe H, Ohtomo Y, Idezuki Y. Safe intraabdominal pressure of carbon dioxide pneumoperitoneum during laparoscopic surgery. Surgery. 1993;114(3):549-54. [Link]
24. Jakimowicz J, Stultiëns G, Smulders F. Laparoscopic insufflation of the abdomen reduces portal venous flow. Surg Endosc. 1998;12(2):129-32. [Link] [DOI:10.1007/s004649900612]
25. Bostanci EB, Yol S, Teke Z, Kayaalp C, Sakaogullari Z, Ozel Turkcu U, et al. Effects of carbon dioxide pneumoperitoneum on hepatic function in obstructive jaundice: An experimental study in a rat model. Langenbecks Arch Surg. 2010;395(6):667-76. [Link] [DOI:10.1007/s00423-009-0577-6]
26. Kotake Y, Takeda J, Matsumoto M, Tagawa M, Kikuchi H. Subclinical hepatic dysfunction in laparoscopic cholecystectomy and laparoscopic colectomy. Br J Anaesth. 2001;87(5):774-7. [Link] [DOI:10.1093/bja/87.5.774]
27. Donmez T, Uzman S, Yildirim D, Hut A, Avaroglu HI, Erdem DA, et al. Is there any effect of pneumoperitoneum pressure on coagulation and fibrinolysis during laparoscopic cholecystectomy?. PeerJ. 2016;4:e2375. [Link] [DOI:10.7717/peerj.2375]
28. Vecchio R, Cacciola E, Martino M, Cacciola RR, MacFadyen BV. Modifications of coagulation and fibrinolytic parameters in laparoscopic cholecystectomy. Surg Endosc. 2003;17(3):428-33. [Link] [DOI:10.1007/s00464-001-8291-7]
29. Giraudo G, Brachet Contul R, Caccetta M, Morino M. Gasless laparoscopy could avoid alterations in hepatic function. Surg Endosc. 2001;15(7):741-6. [Link] [DOI:10.1007/s004640090020]
30. Hasukić S. Postoperative changes in liver function tests: Randomized comparison of low- and high-pressure laparoscopic cholecystectomy. Surg Endosc. 2005;19(11):1451-5. [Link] [DOI:10.1007/s00464-005-0061-5]
31. Morino M, Giraudo G, Festa V. Alterations in hepatic function during laparoscopic surgery. An experimental clinical study. Surg Endosc. 1998;12(7):968-72. [Link] [DOI:10.1007/s004649900758]
32. Tan M, Xu FF, Peng JS, Li DM, Chen LH, Lv BJ, et al. Changes in the level of serum liver enzymes after laparoscopic surgery. World J Gastroenterol. 2003;9(2):364-7. [Link] [DOI:10.3748/wjg.v9.i2.364]








