Volume 6, Issue 2 (2025)
J Clinic Care Skill 2025, 6(2): 97-103 |
Back to browse issues page
Article Type:
Subject:
Ethics code: IR.YUMS.REC.1400.119
History
Received: 2025/05/15 | Accepted: 2025/06/28 | Published: 2025/07/1
Received: 2025/05/15 | Accepted: 2025/06/28 | Published: 2025/07/1
How to cite this article
Fazeli M, Malekzadeh M, Bayatmanesh H, Neysarian P, Arefkhah N, Sedaghattalab M. Effects of Interferon Beta-1 and Dexamethasone on the Treatment of Hospitalized COVID-19 Patients. J Clinic Care Skill 2025; 6 (2) :97-103
URL: http://jccs.yums.ac.ir/article-1-403-en.html
URL: http://jccs.yums.ac.ir/article-1-403-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
M.A. Fazeli1 
 , M. Malekzadeh2
, M. Malekzadeh2 
 , H. Bayatmanesh3
, H. Bayatmanesh3 
 , P. Neysarian4
, P. Neysarian4 
 , N. Arefkhah1
, N. Arefkhah1 
 , M. Sedaghattalab *5
, M. Sedaghattalab *5 


 , M. Malekzadeh2
, M. Malekzadeh2 
 , H. Bayatmanesh3
, H. Bayatmanesh3 
 , P. Neysarian4
, P. Neysarian4 
 , N. Arefkhah1
, N. Arefkhah1 
 , M. Sedaghattalab *5
, M. Sedaghattalab *5 

1- Department of Infectious Disease, Faculty of Medicine, Yasuj University of Medical Sciences, Yasuj, Iran
2- Social Determinant of Health Research Center, Yasuj University of Medical Sciences, Yasuj, Iran
3- “Imam Sajad Hospital” and “Department of Nursing, Faculty of Nursing & Midwifery”, Yasuj University of Medical Sciences, Yasuj, Iran
4- “Shahid Jalil Hospital” and “Department of Midwifery, Faculty of Nursing & Midwifery”, Yasuj University of Medical Sciences, Yasuj, Iran
5- Department of Internal Medicine, Faculty of Medicine, Yasuj University of Medical Sciences, Yasuj, Iran
2- Social Determinant of Health Research Center, Yasuj University of Medical Sciences, Yasuj, Iran
3- “Imam Sajad Hospital” and “Department of Nursing, Faculty of Nursing & Midwifery”, Yasuj University of Medical Sciences, Yasuj, Iran
4- “Shahid Jalil Hospital” and “Department of Midwifery, Faculty of Nursing & Midwifery”, Yasuj University of Medical Sciences, Yasuj, Iran
5- Department of Internal Medicine, Faculty of Medicine, Yasuj University of Medical Sciences, Yasuj, Iran
Full-Text (HTML) (482 Views)
Introduction
The outbreak of atypical pneumonia caused by a new coronavirus in Wuhan, China, dominated world news in December 2019 [1, 2]. The World Health Organization (WHO) named it severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the disease was designated Coronavirus Disease-2019 (COVID-19). Patients with this disease typically present at medical centers with symptoms such as dry cough, fever, sputum production, upper respiratory congestion, shortness of breath, and, less commonly, headache, diarrhea, and hemoptysis [3], as well as decreased smell and taste [4].
Cytokine storms and pulmonary injuries caused by inflammation lead to fatal complications in patients with COVID-19. Acute respiratory distress syndrome (ARDS), septic shock, cardiovascular complications, coagulopathy, and metabolic acidosis are the primary causes of death among these patients [1].
COVID-19 also results in lung tissue injuries due to dysregulated and excessive inflammation. In this context, systemic glucocorticoids such as dexamethasone can reduce inflammation, pulmonary injuries, and the progression to acute respiratory failure and death. Dexamethasone is recommended for severe cases of COVID-19 when patients require respiratory support or oxygen [5]. However, the administration of corticosteroids did not decrease mortality or the length of hospitalization for COVID-19 patients in some studies.
According to a systematic review conducted by Abdelrahman et al. [6], corticosteroids can increase the severity of COVID-19; however, they may be beneficial when used in combination with antiviral drugs. Based on the results of this study, dexamethasone, a corticosteroid recently approved by the FDA for the treatment of COVID-19, was not significantly associated with a lower incidence of complications, mortality, or clinical improvement compared to standard care [6].
Tomazini et al. reported that dexamethasone can reduce the risk of mortality compared to other corticosteroids or standard care [7]; however, Sing et al. stated that the rationale for using corticosteroids remains controversial due to their high risk of adverse effects [8]. Interferons (IFNs) play key roles in reducing inflammation and regulating the immune system. They are most effective when administered immediately after COVID-19 infection; however, if prescribed with a delay, they become ineffective in controlling the proliferation of the virus [1]. Clinical studies have also produced varying results regarding the effectiveness of type I IFNs, including interferons alpha and beta, in the treatment of SARS-CoV [9, 10]. Previous experience with the treatment of SARS and MERS indicates that multiple interventions, including antiviral drugs such as lopinavir/ritonavir and umifenovir, corticosteroids, IFNs, ribavirin, and newly introduced drugs, such as chloroquine, hydroxychloroquine, and dexamethasone, may lead to clinical improvement in COVID-19 patients. Nevertheless, the results and relevant data remain inconclusive.
Despite the urgent need for effective treatments to combat the COVID-19 pandemic, no definitive treatment has been established to date, and treatment guidelines vary across different countries. Given the importance of the COVID-19 pandemic and the inconclusive nature of the relevant data, as well as the lack of evidence regarding the effectiveness and safety of such treatments, the present study aimed to determine the effect of interferon-β (IFN-β) on mortality, length of hospitalization, and clinical status in patients with COVID-19.
Materials and Methods
Study design and patients
This phase 2, open-label, randomized trial examined adult patients aged at least 18 years who were admitted to Shahid Jalil and Shahid Beheshti hospitals in Yasuj from October 23, 2021, to May 22, 2022, due to confirmed COVID-19 virology.
Inclusion and exclusion criteria
The inclusion criteria for the study were hospitalization with a definitive diagnosis of COVID-19 made by an infectious disease specialist, a positive PCR test or spiral lung CT scan, being at least 18 years of age, and having a glomerular filtration rate (GFR) above 30%. The exclusion criteria included a history of pancytopenia or increased liver enzymes exceeding 10 times the normal level, pregnancy in women, severe forms of the disease requiring transfer to intensive care units, and patients who had taken immunosuppressive or antiviral drugs.
Sample size
Given the lack of studies in this field, a sample size of 172 was determined with a ratio of 1:1 per group, using α=0.05, an effect size of 0.50, and power of 0.90, with G*Power 3.1.9.7 software. The total sample size was increased to 176 to account for potential dropouts.
Randomization and masking
Patients were randomly assigned to intervention group 1, which received remdesivir, interferon beta-1, and dexamethasone, and intervention group 2, which received remdesivir and dexamethasone, in a ratio of 1:1 using block randomization. A serial number was assigned to each patient by the coordinator, with each serial number corresponding to a randomization list that determined the research groups. The drugs were obtained from the hospital pharmacy and were administered to the patients by the ward nurses.
Procedure
Remdesivir was administered at a dose of 200mg as an initial dose, followed by 100mg daily for 5 days. IFN-β-1a was given at a dose of one ampoule subcutaneously every other day for a total of 4 doses (each 30-µg vial of interferon is equivalent to 6 million IU), and dexamethasone was administered at a dose of 8mg daily intravenously. Patients in intervention group 2 received remdesivir and dexamethasone, with remdesivir administered at a dose of 200mg as an initial dose, followed by 100mg daily for 5 days, and dexamethasone intravenously at a dose of 8mg daily.
Clinical and laboratory monitoring
Clinical results, including patient history and physical examinations, as well as laboratory and radiological findings, were recorded in the patients’ electronic files and the pre-designed database. Chest X-rays and ECGs were initially obtained from patients over 50 years of age with underlying cardiovascular diseases and were repeated at regular intervals as needed. Patients with underlying heart disease underwent cardiac monitoring. Depending on the patients’ condition, supportive measures such as oxygen masks were provided if necessary.
High-resolution CT scans were performed on the patients, and all were followed up at the infectious disease clinic one week after discharge and then weekly for one month. The early diagnosis of SARS-CoV-2 infection was confirmed upon admission. All admitted patients had to have laboratory-confirmed SARS-CoV-2 infection through RT-PCR on a nasopharyngeal swab.
The complete blood count (CBC), liver and kidney function tests, BUN and creatinine (Cr), lactate dehydrogenase (LDH), creatine kinase (CK), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and interleukin 6 (IL-6) levels were regularly monitored until discharge. For patients presenting symptoms of urinary tract infection, blood and urine samples were taken for bacterial culture based on clinical indications.
Outcomes
The mean arterial blood oxygen saturation levels of both groups were compared during admission and discharge, and the length of hospital stay and mortality rates of both groups were compared at the end of the study.
Statistical analysis
Data analysis was conducted using SPSS version 25. The Chi-square test was used to compare categorical parameters, the independent t-test was employed for continuous parameters, and the paired t-test was used for intra-group comparisons. A significance level of 0.05 was considered.
Findings
A total of 662 patients with COVID-19 were admitted to the hospital from October 23, 2021, to May 22, 2022. Of these, 486 patients were excluded from the study (32 patients under 18 years of age, 42 patients due to pregnancy, 42 patients with GFR<30%, 21 patients with pancytopenia, 22 patients with liver enzyme issues, 87 patients requiring transfer to other wards, 162 patients using broad-spectrum or immunosuppressive drugs, and 78 patients who did not give consent to participate in the study). Ultimately, 176 individuals participated in the study. One patient in the remdesivir and dexamethasone group was also excluded from the intervention due to transfer to other medical centers (Figure 1).
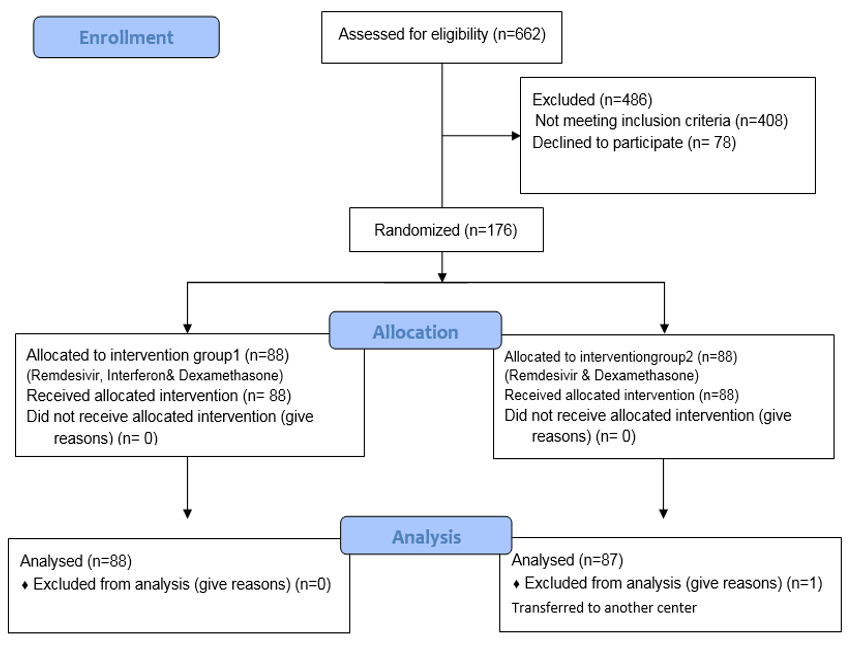
Figure 1. Flow diagram of the study.
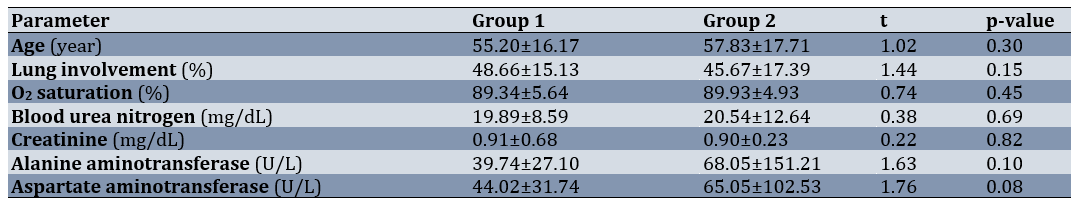
The mean participants’ age was 56.51±16.96 years. Diabetes was the most common underlying disease in group 1 (n=10) and in group 2 (n=11). Regarding gender, in group 1, there were 48 females (54.5%) and 40 males (45.5%), while in group 2, there were 40 females (45.5%) and 44 males (50.6%), respectively (χ2=0.46; p=0.54). In terms of underlying diseases, group 1 included 35 participants (39.8%) with underlying conditions and 53 participants (60.2%) without. In group 2, there were 25 participants (28.7%) with underlying conditions and 62 participants (71.3%) without, respectively (χ2=2.36; p=0.15). The two groups were similar and showed no significant differences in terms of demographic characteristics, extent of lung involvement, presence or absence of underlying disease, percentage of oxygen saturation, and laboratory tests (BUN, Cr, alanine aminotransferase (ALT), and aspartate aminotransferase (AST)) at baseline (p>0.05; Table 1).
Table 1. Comparison of the patients’ demographic characteristics between the two groups and some laboratory indices on admission
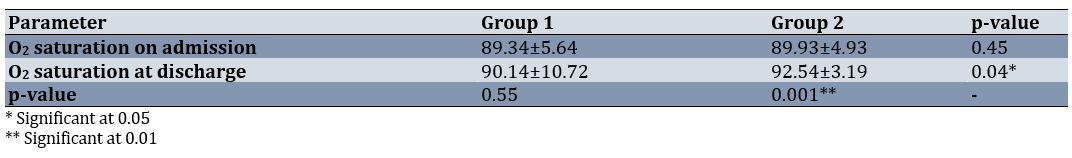
The mean percentage of arterial blood saturation was significantly different between the two groups at the time of discharge from the hospital (95% CI: 4.78-0.031, p=0.047; Table 2). The paired t-test indicated no significant difference in the mean O2 saturation at the time of admission and discharge in group 1, while the difference was significant in group 2 (95% CI: 3.48-1.79, p=0.001; Table 2).
Table 2. Comparison of the mean oxygen saturation of groups at the time of admission and discharge
The mean length of hospitalization in the two groups (7.02±2.56 days in group 1 vs. 5.73±2.04 days in group 2) showed a significant difference (95% CI: 0.59-1.97; t=3.36; p=0.001). Both groups exhibited a significant difference in terms of the number of deceased patients. Regarding mortality, in group 1, there were 7 deaths (8.0%) and 81 discharges (92.0%), while in group 2, there were 81 deaths (92.0%) and 6 discharges (8.0%) (χ²=7.20; p=0.007).
Discussion
This study aimed to determine the effect of IFN-β on mortality, length of hospitalization, and clinical status in patients with COVID-19. Interferon therapy has previously been employed in the treatment of SARS and MERS. IFN-β has exhibited antiviral effects against SARS-CoV in initial in vitro studies [11]. The antiviral effects of IFN-β against MERS-CoV were found to be higher than those of lopinavir-ritonavir in animal studies [12].
The administration of remdesivir and dexamethasone, compared to remdesivir, interferon, and dexamethasone, decreased the length of hospitalization, improved oxygenation, and reduced mortality.
In a double-blind study involving hospitalized COVID-19 patients, Kalil et al. showed that patients requiring high-dose oxygen in the interferon group have a worse prognosis and experience worsened respiratory status. They reported that the interferon and remdesivir group did not achieve greater clinical benefits than the remdesivir group [13].
In a study conducted by Ader et al., 583 hospitalized COVID-19 patients were allocated to four groups; lopinavir/ritonavir plus IFN-β-1a (44µg on days 1, 3, and 6; n=145), hydroxychloroquine (400mg for 9 days; n=145), lopinavir/ritonavir (400/100mg BD for 2 weeks; n=145), and a control group (n=148). None of the treatments leads to clinical improvement in the patients or the removal of the virus from their respiratory samples. They also reported no additional benefit from the use of IFN-β-1a [14].
In a study by Khamis et al., hospitalized patients with moderate to severe COVID-19 were treated with a combination of Favipiravir and inhaled IFN-β-1a (n=44) or hydroxychloroquine (n=45). They reported that there is no significant difference between the two groups in terms of inflammatory biomarkers, length of hospitalization, ICU admission, and mortality rates [15]. The results of all the studies were consistent with our findings.
Corticosteroids, like dexamethasone, have extensive effects on both innate and adaptive immunity. Adaptive immunity may be essential in the immunopathology of COVID-19, as the onset of ARDS is temporally related to the emergence of specific antibodies against SARS-CoV-2 [16].
According to Noreen et al., dexamethasone is considered a “major development” in the fight against COVID-19 in the current pandemic. Steroidal dexamethasone has been presented as a significant advancement that markedly reduces the mortality rate among severe cases of COVID-19 [17].
Although the underlying mechanisms of the beneficial effects of dexamethasone during COVID-19 remain unclear, research by Sinha et al. indicated that dexamethasone affects circulating neutrophils, alters IFN active neutrophils, downregulates interferon-stimulated genes (ISGs), and activates IL-1R2+ neutrophils in severe COVID-19 cases. In addition to expanding immunosuppressive immature neutrophils, dexamethasone transforms neutrophils from information receptors to information providers, thereby restoring cell interactions [18].
Arabi et al. indicated that the combination of IFNs with ribavirin does not improve the disease in patients with MERS [19]. However, in a study conducted by Davoudi-Monfared et al., 42 patients with severe COVID-19 received subcutaneous IFN-β-1a three times a week (12million IU/ml) for two consecutive weeks, while the control group received standard treatment with hydroxychloroquine (400mg twice a day [BID] on the first day and then 200mg BID) and lopinavir-ritonavir (400mg and 100mg BID, respectively) for 7 to 10 days. They found that the administration of IFN-β-1a in the early stages of the disease increases the discharge rate of patients and decreases their mortality. Glucocorticoids and antibiotics were also used in both groups as needed, based on hospital protocols [20].
In another study by Alavi Darazam et al., 60 hospitalized patients with severe COVID-19 were randomly assigned to three groups of 20 patients each. One group received IFN-β-1a (12,000IU subcutaneously on days 1, 3, and 6), hydroxychloroquine, and Kaletra in the early phase of the disease; the second group received IFN-β-1b (8,000,000IU subcutaneously on days 1, 3, and 6), hydroxychloroquine, and Kaletra; and the control group received hydroxychloroquine (a single dose of 400mg on day 1, orally) and Kaletra (lopinavir/ritonavir; 400mg/100mg orally twice a day for 10 days). They concluded that the administration of IFN-β-1a accelerates the recovery of patients [21]. IFNs cause host cells to shift to antiviral mode, thereby inhibiting the reproduction and spread of viruses through the expression of multiple genes. Although the three main groups of IFNs include types I, II, and III, type I plays an essential role in antiviral responses and immune system regulation. A reduction in interferon levels and type I interferon genes leads to a decreased immune response.
SARS-CoV and MERS-CoV decrease the expression of interferon genes and the immune response. Given the similarity between COVID-19 and these two previous viruses, SARS-CoV-2 likely inhibits type I interferon in the early phase of the disease [22, 23]. In a study by Monk et al., 39 hospitalized patients with COVID-19 were treated daily with inhaled IFN-β-1a (administered via nebulizer) for 2 weeks, and their results indicated that faster recovery is observed in the intervention group [24]. Contrary to the results of these studies, in our study, oxygenation improved, and the length of hospitalization and mortality rate decreased in the remdesivir and dexamethasone group compared to the “interferon, remdesivir, and dexamethasone” group. The low sample sizes of most of the aforementioned studies and their consequently low power may have contributed to this inconsistency.
IFNs are natural cytokines produced in response to viral infections. They activate ISGs and increase the expression of angiotensin-converting enzyme 2 (ACE2). They can protect lung cells against damage caused by COVID-19 by deactivating angiotensin 2 [25].
Salto-Alejandre et al. indicated that there is no relationship between IFN-β administration and lower mortality in hospitalized COVID-19 patients. They also stated that further research should be conducted to determine whether IFN-β may be useful in the early stages of the disease or in specific subgroups of patients [26].
Our findings confirmed the results of the WHO SOLIDARITY trial, indicating that interferon is not effective in the recovery of hospitalized patients with COVID-19 [27].
Like numerous studies, the present study also had limitations, such as the complexity of blinded therapies and the necessity of initiating the trial as soon as possible, which led to the selection of an open-label design. Finally, the clinical use of dexamethasone and its role in the management of COVID-19 require further clinical evidence and research.
Conclusion
The administration of remdesivir and dexamethasone in hospitalized COVID-19 patients decreases the length of hospitalization, improves O2 saturation, and reduces mortality.
Acknowledgments: We are grateful to all patients who participated in the present study.
Ethical Permissions: This study was approved by the Ethics Committee in Biomedical Research of Yasuj University of Medical Sciences with the code IR.YUMS.REC.1400.119. All patients provided informed consent to participate in the study. The present study was registered in the Iranian Registry of Clinical Trials (IRCT), a member of the international centers approved by the World Health Organization, with the internet address https://www.irct.ir and the code IRCT20150622022869N9.
Conflicts of Interests: The authors declared no conflicts of interests.
Authors' Contribution: Fazeli MA (First Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (20%); Malekzadeh M (Second Author), Assistant Researcher/Discussion Writer/Statistical Analyst (15%); Bayatmanesh H (Third Author), Introduction Writer/Methodologist/Assistant Researcher (15%); Neysarian P (Fourth Author), Introduction Writer/Assistant Researcher/Discussion Writer/Statistical Analyst (15%); Arefkhah N (Fifth Author), Introduction Writer/Methodologist/Assistant Researcher (15%); Sedaghattalab M (Sixth Author), Assistant Researcher/Discussion Writer/Statistical Analyst (20%)
Funding/Support: The funders played no role in the study design, data collection, data analysis, data interpretation, or report writing. The corresponding author had full access to all research data and held the final responsibility for making decisions regarding the submission of the manuscript for publication.
The outbreak of atypical pneumonia caused by a new coronavirus in Wuhan, China, dominated world news in December 2019 [1, 2]. The World Health Organization (WHO) named it severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the disease was designated Coronavirus Disease-2019 (COVID-19). Patients with this disease typically present at medical centers with symptoms such as dry cough, fever, sputum production, upper respiratory congestion, shortness of breath, and, less commonly, headache, diarrhea, and hemoptysis [3], as well as decreased smell and taste [4].
Cytokine storms and pulmonary injuries caused by inflammation lead to fatal complications in patients with COVID-19. Acute respiratory distress syndrome (ARDS), septic shock, cardiovascular complications, coagulopathy, and metabolic acidosis are the primary causes of death among these patients [1].
COVID-19 also results in lung tissue injuries due to dysregulated and excessive inflammation. In this context, systemic glucocorticoids such as dexamethasone can reduce inflammation, pulmonary injuries, and the progression to acute respiratory failure and death. Dexamethasone is recommended for severe cases of COVID-19 when patients require respiratory support or oxygen [5]. However, the administration of corticosteroids did not decrease mortality or the length of hospitalization for COVID-19 patients in some studies.
According to a systematic review conducted by Abdelrahman et al. [6], corticosteroids can increase the severity of COVID-19; however, they may be beneficial when used in combination with antiviral drugs. Based on the results of this study, dexamethasone, a corticosteroid recently approved by the FDA for the treatment of COVID-19, was not significantly associated with a lower incidence of complications, mortality, or clinical improvement compared to standard care [6].
Tomazini et al. reported that dexamethasone can reduce the risk of mortality compared to other corticosteroids or standard care [7]; however, Sing et al. stated that the rationale for using corticosteroids remains controversial due to their high risk of adverse effects [8]. Interferons (IFNs) play key roles in reducing inflammation and regulating the immune system. They are most effective when administered immediately after COVID-19 infection; however, if prescribed with a delay, they become ineffective in controlling the proliferation of the virus [1]. Clinical studies have also produced varying results regarding the effectiveness of type I IFNs, including interferons alpha and beta, in the treatment of SARS-CoV [9, 10]. Previous experience with the treatment of SARS and MERS indicates that multiple interventions, including antiviral drugs such as lopinavir/ritonavir and umifenovir, corticosteroids, IFNs, ribavirin, and newly introduced drugs, such as chloroquine, hydroxychloroquine, and dexamethasone, may lead to clinical improvement in COVID-19 patients. Nevertheless, the results and relevant data remain inconclusive.
Despite the urgent need for effective treatments to combat the COVID-19 pandemic, no definitive treatment has been established to date, and treatment guidelines vary across different countries. Given the importance of the COVID-19 pandemic and the inconclusive nature of the relevant data, as well as the lack of evidence regarding the effectiveness and safety of such treatments, the present study aimed to determine the effect of interferon-β (IFN-β) on mortality, length of hospitalization, and clinical status in patients with COVID-19.
Materials and Methods
Study design and patients
This phase 2, open-label, randomized trial examined adult patients aged at least 18 years who were admitted to Shahid Jalil and Shahid Beheshti hospitals in Yasuj from October 23, 2021, to May 22, 2022, due to confirmed COVID-19 virology.
Inclusion and exclusion criteria
The inclusion criteria for the study were hospitalization with a definitive diagnosis of COVID-19 made by an infectious disease specialist, a positive PCR test or spiral lung CT scan, being at least 18 years of age, and having a glomerular filtration rate (GFR) above 30%. The exclusion criteria included a history of pancytopenia or increased liver enzymes exceeding 10 times the normal level, pregnancy in women, severe forms of the disease requiring transfer to intensive care units, and patients who had taken immunosuppressive or antiviral drugs.
Sample size
Given the lack of studies in this field, a sample size of 172 was determined with a ratio of 1:1 per group, using α=0.05, an effect size of 0.50, and power of 0.90, with G*Power 3.1.9.7 software. The total sample size was increased to 176 to account for potential dropouts.
Randomization and masking
Patients were randomly assigned to intervention group 1, which received remdesivir, interferon beta-1, and dexamethasone, and intervention group 2, which received remdesivir and dexamethasone, in a ratio of 1:1 using block randomization. A serial number was assigned to each patient by the coordinator, with each serial number corresponding to a randomization list that determined the research groups. The drugs were obtained from the hospital pharmacy and were administered to the patients by the ward nurses.
Procedure
Remdesivir was administered at a dose of 200mg as an initial dose, followed by 100mg daily for 5 days. IFN-β-1a was given at a dose of one ampoule subcutaneously every other day for a total of 4 doses (each 30-µg vial of interferon is equivalent to 6 million IU), and dexamethasone was administered at a dose of 8mg daily intravenously. Patients in intervention group 2 received remdesivir and dexamethasone, with remdesivir administered at a dose of 200mg as an initial dose, followed by 100mg daily for 5 days, and dexamethasone intravenously at a dose of 8mg daily.
Clinical and laboratory monitoring
Clinical results, including patient history and physical examinations, as well as laboratory and radiological findings, were recorded in the patients’ electronic files and the pre-designed database. Chest X-rays and ECGs were initially obtained from patients over 50 years of age with underlying cardiovascular diseases and were repeated at regular intervals as needed. Patients with underlying heart disease underwent cardiac monitoring. Depending on the patients’ condition, supportive measures such as oxygen masks were provided if necessary.
High-resolution CT scans were performed on the patients, and all were followed up at the infectious disease clinic one week after discharge and then weekly for one month. The early diagnosis of SARS-CoV-2 infection was confirmed upon admission. All admitted patients had to have laboratory-confirmed SARS-CoV-2 infection through RT-PCR on a nasopharyngeal swab.
The complete blood count (CBC), liver and kidney function tests, BUN and creatinine (Cr), lactate dehydrogenase (LDH), creatine kinase (CK), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and interleukin 6 (IL-6) levels were regularly monitored until discharge. For patients presenting symptoms of urinary tract infection, blood and urine samples were taken for bacterial culture based on clinical indications.
Outcomes
The mean arterial blood oxygen saturation levels of both groups were compared during admission and discharge, and the length of hospital stay and mortality rates of both groups were compared at the end of the study.
Statistical analysis
Data analysis was conducted using SPSS version 25. The Chi-square test was used to compare categorical parameters, the independent t-test was employed for continuous parameters, and the paired t-test was used for intra-group comparisons. A significance level of 0.05 was considered.
Findings
A total of 662 patients with COVID-19 were admitted to the hospital from October 23, 2021, to May 22, 2022. Of these, 486 patients were excluded from the study (32 patients under 18 years of age, 42 patients due to pregnancy, 42 patients with GFR<30%, 21 patients with pancytopenia, 22 patients with liver enzyme issues, 87 patients requiring transfer to other wards, 162 patients using broad-spectrum or immunosuppressive drugs, and 78 patients who did not give consent to participate in the study). Ultimately, 176 individuals participated in the study. One patient in the remdesivir and dexamethasone group was also excluded from the intervention due to transfer to other medical centers (Figure 1).

Figure 1. Flow diagram of the study.
The mean participants’ age was 56.51±16.96 years. Diabetes was the most common underlying disease in group 1 (n=10) and in group 2 (n=11). Regarding gender, in group 1, there were 48 females (54.5%) and 40 males (45.5%), while in group 2, there were 40 females (45.5%) and 44 males (50.6%), respectively (χ2=0.46; p=0.54). In terms of underlying diseases, group 1 included 35 participants (39.8%) with underlying conditions and 53 participants (60.2%) without. In group 2, there were 25 participants (28.7%) with underlying conditions and 62 participants (71.3%) without, respectively (χ2=2.36; p=0.15). The two groups were similar and showed no significant differences in terms of demographic characteristics, extent of lung involvement, presence or absence of underlying disease, percentage of oxygen saturation, and laboratory tests (BUN, Cr, alanine aminotransferase (ALT), and aspartate aminotransferase (AST)) at baseline (p>0.05; Table 1).
Table 1. Comparison of the patients’ demographic characteristics between the two groups and some laboratory indices on admission

The mean percentage of arterial blood saturation was significantly different between the two groups at the time of discharge from the hospital (95% CI: 4.78-0.031, p=0.047; Table 2). The paired t-test indicated no significant difference in the mean O2 saturation at the time of admission and discharge in group 1, while the difference was significant in group 2 (95% CI: 3.48-1.79, p=0.001; Table 2).
Table 2. Comparison of the mean oxygen saturation of groups at the time of admission and discharge

The mean length of hospitalization in the two groups (7.02±2.56 days in group 1 vs. 5.73±2.04 days in group 2) showed a significant difference (95% CI: 0.59-1.97; t=3.36; p=0.001). Both groups exhibited a significant difference in terms of the number of deceased patients. Regarding mortality, in group 1, there were 7 deaths (8.0%) and 81 discharges (92.0%), while in group 2, there were 81 deaths (92.0%) and 6 discharges (8.0%) (χ²=7.20; p=0.007).
Discussion
This study aimed to determine the effect of IFN-β on mortality, length of hospitalization, and clinical status in patients with COVID-19. Interferon therapy has previously been employed in the treatment of SARS and MERS. IFN-β has exhibited antiviral effects against SARS-CoV in initial in vitro studies [11]. The antiviral effects of IFN-β against MERS-CoV were found to be higher than those of lopinavir-ritonavir in animal studies [12].
The administration of remdesivir and dexamethasone, compared to remdesivir, interferon, and dexamethasone, decreased the length of hospitalization, improved oxygenation, and reduced mortality.
In a double-blind study involving hospitalized COVID-19 patients, Kalil et al. showed that patients requiring high-dose oxygen in the interferon group have a worse prognosis and experience worsened respiratory status. They reported that the interferon and remdesivir group did not achieve greater clinical benefits than the remdesivir group [13].
In a study conducted by Ader et al., 583 hospitalized COVID-19 patients were allocated to four groups; lopinavir/ritonavir plus IFN-β-1a (44µg on days 1, 3, and 6; n=145), hydroxychloroquine (400mg for 9 days; n=145), lopinavir/ritonavir (400/100mg BD for 2 weeks; n=145), and a control group (n=148). None of the treatments leads to clinical improvement in the patients or the removal of the virus from their respiratory samples. They also reported no additional benefit from the use of IFN-β-1a [14].
In a study by Khamis et al., hospitalized patients with moderate to severe COVID-19 were treated with a combination of Favipiravir and inhaled IFN-β-1a (n=44) or hydroxychloroquine (n=45). They reported that there is no significant difference between the two groups in terms of inflammatory biomarkers, length of hospitalization, ICU admission, and mortality rates [15]. The results of all the studies were consistent with our findings.
Corticosteroids, like dexamethasone, have extensive effects on both innate and adaptive immunity. Adaptive immunity may be essential in the immunopathology of COVID-19, as the onset of ARDS is temporally related to the emergence of specific antibodies against SARS-CoV-2 [16].
According to Noreen et al., dexamethasone is considered a “major development” in the fight against COVID-19 in the current pandemic. Steroidal dexamethasone has been presented as a significant advancement that markedly reduces the mortality rate among severe cases of COVID-19 [17].
Although the underlying mechanisms of the beneficial effects of dexamethasone during COVID-19 remain unclear, research by Sinha et al. indicated that dexamethasone affects circulating neutrophils, alters IFN active neutrophils, downregulates interferon-stimulated genes (ISGs), and activates IL-1R2+ neutrophils in severe COVID-19 cases. In addition to expanding immunosuppressive immature neutrophils, dexamethasone transforms neutrophils from information receptors to information providers, thereby restoring cell interactions [18].
Arabi et al. indicated that the combination of IFNs with ribavirin does not improve the disease in patients with MERS [19]. However, in a study conducted by Davoudi-Monfared et al., 42 patients with severe COVID-19 received subcutaneous IFN-β-1a three times a week (12million IU/ml) for two consecutive weeks, while the control group received standard treatment with hydroxychloroquine (400mg twice a day [BID] on the first day and then 200mg BID) and lopinavir-ritonavir (400mg and 100mg BID, respectively) for 7 to 10 days. They found that the administration of IFN-β-1a in the early stages of the disease increases the discharge rate of patients and decreases their mortality. Glucocorticoids and antibiotics were also used in both groups as needed, based on hospital protocols [20].
In another study by Alavi Darazam et al., 60 hospitalized patients with severe COVID-19 were randomly assigned to three groups of 20 patients each. One group received IFN-β-1a (12,000IU subcutaneously on days 1, 3, and 6), hydroxychloroquine, and Kaletra in the early phase of the disease; the second group received IFN-β-1b (8,000,000IU subcutaneously on days 1, 3, and 6), hydroxychloroquine, and Kaletra; and the control group received hydroxychloroquine (a single dose of 400mg on day 1, orally) and Kaletra (lopinavir/ritonavir; 400mg/100mg orally twice a day for 10 days). They concluded that the administration of IFN-β-1a accelerates the recovery of patients [21]. IFNs cause host cells to shift to antiviral mode, thereby inhibiting the reproduction and spread of viruses through the expression of multiple genes. Although the three main groups of IFNs include types I, II, and III, type I plays an essential role in antiviral responses and immune system regulation. A reduction in interferon levels and type I interferon genes leads to a decreased immune response.
SARS-CoV and MERS-CoV decrease the expression of interferon genes and the immune response. Given the similarity between COVID-19 and these two previous viruses, SARS-CoV-2 likely inhibits type I interferon in the early phase of the disease [22, 23]. In a study by Monk et al., 39 hospitalized patients with COVID-19 were treated daily with inhaled IFN-β-1a (administered via nebulizer) for 2 weeks, and their results indicated that faster recovery is observed in the intervention group [24]. Contrary to the results of these studies, in our study, oxygenation improved, and the length of hospitalization and mortality rate decreased in the remdesivir and dexamethasone group compared to the “interferon, remdesivir, and dexamethasone” group. The low sample sizes of most of the aforementioned studies and their consequently low power may have contributed to this inconsistency.
IFNs are natural cytokines produced in response to viral infections. They activate ISGs and increase the expression of angiotensin-converting enzyme 2 (ACE2). They can protect lung cells against damage caused by COVID-19 by deactivating angiotensin 2 [25].
Salto-Alejandre et al. indicated that there is no relationship between IFN-β administration and lower mortality in hospitalized COVID-19 patients. They also stated that further research should be conducted to determine whether IFN-β may be useful in the early stages of the disease or in specific subgroups of patients [26].
Our findings confirmed the results of the WHO SOLIDARITY trial, indicating that interferon is not effective in the recovery of hospitalized patients with COVID-19 [27].
Like numerous studies, the present study also had limitations, such as the complexity of blinded therapies and the necessity of initiating the trial as soon as possible, which led to the selection of an open-label design. Finally, the clinical use of dexamethasone and its role in the management of COVID-19 require further clinical evidence and research.
Conclusion
The administration of remdesivir and dexamethasone in hospitalized COVID-19 patients decreases the length of hospitalization, improves O2 saturation, and reduces mortality.
Acknowledgments: We are grateful to all patients who participated in the present study.
Ethical Permissions: This study was approved by the Ethics Committee in Biomedical Research of Yasuj University of Medical Sciences with the code IR.YUMS.REC.1400.119. All patients provided informed consent to participate in the study. The present study was registered in the Iranian Registry of Clinical Trials (IRCT), a member of the international centers approved by the World Health Organization, with the internet address https://www.irct.ir and the code IRCT20150622022869N9.
Conflicts of Interests: The authors declared no conflicts of interests.
Authors' Contribution: Fazeli MA (First Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (20%); Malekzadeh M (Second Author), Assistant Researcher/Discussion Writer/Statistical Analyst (15%); Bayatmanesh H (Third Author), Introduction Writer/Methodologist/Assistant Researcher (15%); Neysarian P (Fourth Author), Introduction Writer/Assistant Researcher/Discussion Writer/Statistical Analyst (15%); Arefkhah N (Fifth Author), Introduction Writer/Methodologist/Assistant Researcher (15%); Sedaghattalab M (Sixth Author), Assistant Researcher/Discussion Writer/Statistical Analyst (20%)
Funding/Support: The funders played no role in the study design, data collection, data analysis, data interpretation, or report writing. The corresponding author had full access to all research data and held the final responsibility for making decisions regarding the submission of the manuscript for publication.
Keywords:
References
1. Tsang HF, Chan LWC, Cho WCS, Yu ACS, Yim AKY, Chan AKC, et al. An update on COVID-19 pandemic: The epidemiology, pathogenesis, prevention and treatment strategies. Expert Rev Anti Infect Ther. 2021;19(7):877-88. [Link] [DOI:10.1080/14787210.2021.1863146]
2. Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9(1):221-36. [Link] [DOI:10.1080/22221751.2020.1719902]
3. Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730-41. [Link] [DOI:10.1111/all.14238]
4. Spinato G, Fabbris C, Polesel J, Cazzador D, Borsetto D, Hopkins C, et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA. 2020;323(20):2089-90. [Link] [DOI:10.1001/jama.2020.6771]
5. RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693-704. [Link] [DOI:10.1056/NEJMoa2021436]
6. Abdelrahman Z, Liu Q, Jiang S, Li M, Sun Q, Zhang Y, et al. Evaluation of the current therapeutic approaches for COVID-19: A systematic review and a meta-analysis. Front Pharmacol. 2021;12:607408. [Link] [DOI:10.3389/fphar.2021.607408]
7. Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: The CoDEX randomized clinical trial. JAMA. 2020;324(13):1307-16. [Link] [DOI:10.1001/jama.2020.17021]
8. Singh AK, Majumdar S, Singh R, Misra A. Role of corticosteroid in the management of COVID-19: A systemic review and a Clinician's perspective. Diabetes Metab Syndr. 2020;14(5):971-8. [Link] [DOI:10.1016/j.dsx.2020.06.054]
9. Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, Chan KS, et al. Role of lopinavir/ritonavir in the treatment of SARS: Initial virological and clinical findings. Thorax. 2004;59(3):252-6. [Link] [DOI:10.1136/thorax.2003.012658]
10. Zhao Z, Zhang F, Xu M, Huang K, Zhong W, Cai W, et al. Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou, PR China. J Med Microbiol. 2003;52(Pt 8):715-20. [Link] [DOI:10.1099/jmm.0.05320-0]
11. Scagnolari C, Vicenzi E, Bellomi F, Stillitano MG, Pinna D, Poli G, et al. Increased sensitivity of SARS-coronavirus to a combination of human type I and type II interferons. Antivir Ther. 2004;9(6):1003-11. [Link] [DOI:10.1177/135965350400900618]
12. Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11(1):222. [Link] [DOI:10.1038/s41467-019-13940-6]
13. Kalil AC, Mehta AK, Patterson TF, Erdmann N, Gomez CA, Jain MK, et al. Efficacy of interferon beta-1a plus remdesivir compared with remdesivir alone in hospitalised adults with COVID-19: A double-bind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021;9(12):1365-76. [Link] [DOI:10.1016/S2213-2600(21)00384-2]
14. Ader F, Peiffer-Smadja N, Poissy J, Bouscambert-Duchamp M, Belhadi D, Diallo A, et al. An open-label randomized controlled trial of the effect of lopinavir/ritonavir, lopinavir/ritonavir plus IFN-β-1a and hydroxychloroquine in hospitalized patients with COVID-19. Clin Microbiol Infect. 2021;27(12):1826-37. [Link] [DOI:10.1016/j.cmi.2021.05.020]
15. Khamis F, Al Naabi H, Al Lawati A, Ambusaidi Z, Al Sharji M, Al Barwani U, et al. Randomized controlled open label trial on the use of favipiravir combined with inhaled interferon beta-1b in hospitalized patients with moderate to severe COVID-19 pneumonia. Int J Infect Dis. 2021;102:538-43. [Link] [DOI:10.1016/j.ijid.2020.11.008]
16. Lee YH, Hong CM, Kim DH, Lee TH, Lee J. Clinical course of asymptomatic and mildly symptomatic patients with coronavirus disease admitted to community treatment centers, South Korea. Emerg Infect Dis. 2020;26(10):2346-52. [Link] [DOI:10.3201/eid2610.201620]
17. Noreen S, Maqbool I, Madni A. Dexamethasone: Therapeutic potential, risks, and future projection during COVID-19 pandemic. Eur J Pharmacol. 2021;894:173854. [Link] [DOI:10.1016/j.ejphar.2021.173854]
18. Sinha S, Rosin NL, Arora R, Labit E, Jaffer A, Cao L, et al. Dexamethasone modulates immature neutrophils and interferon programming in severe COVID-19. Nat Med. 2022;28(1):201-11. [Link] [DOI:10.1038/s41591-021-01576-3]
19. Arabi YM, Shalhoub S, Mandourah Y, Al-Hameed F, Al-Omari A, Al Qasim E, et al. Ribavirin and interferon therapy for critically ill patients with middle east respiratory syndrome: A multicenter observational study. Clin Infect Dis. 2020;70(9):1837-44. [Link] [DOI:10.1093/cid/ciz544]
20. Davoudi-Monfared E, Rahmani H, Khalili H, Hajiabdolbaghi M, Salehi M, Abbasian L, et al. A randomized clinical trial of the efficacy and safety of interferon β-1a in treatment of severe COVID-19. Antimicrob Agents Chemother. 2020;64(9):e01061-20. [Link] [DOI:10.1128/AAC.01061-20]
21. Alavi Darazam I, Shokouhi S, Pourhoseingholi MA, Naghibi Irvani SS, Mokhtari M, Shabani M, et al. Role of interferon therapy in severe COVID-19: The COVIFERON randomized controlled trial. Sci Rep. 2021;11(1):8059. [Link] [DOI:10.1038/s41598-021-86859-y]
22. Durbin JE, Fernandez-Sesma A, Lee CK, Rao TD, Frey AB, Moran TM, et al. Type I IFN modulates innate and specific antiviral immunity. J Immunol. 2000;164(8):4220-8. [Link] [DOI:10.4049/jimmunol.164.8.4220]
23. Sallard E, Lescure FX, Yazdanpanah Y, Mentre F, Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. 2020;178:104791. [Link] [DOI:10.1016/j.antiviral.2020.104791]
24. Monk PD, Marsden RJ, Tear VJ, Brookes J, Batten TN, Mankowski M, et al. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med. 2021;9(2):196-206. [Link] [DOI:10.1016/S2213-2600(20)30511-7]
25. Su S, Jiang S. A suspicious role of interferon in the pathogenesis of SARS-CoV-2 by enhancing expression of ACE2. Signal Transduct Target Ther. 2020;5(1):71. [Link] [DOI:10.1038/s41392-020-0185-z]
26. Salto-Alejandre S, Palacios-Baena ZR, Arribas JR, Berenguer J, Carratalà J, Jarrín I, et al. Impact of early interferon-β treatment on the prognosis of patients with COVID-19 in the first wave: A post hoc analysis from a multicenter cohort. Biomed Pharmacother. 2022;146:112572. [Link] [DOI:10.1016/j.biopha.2021.112572]
27. WHO Solidarity Trial Consortium, Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, et al. Repurposed antiviral drugs for Covid-19-interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497-511. [Link] [DOI:10.1056/NEJMoa2023184]








