Volume 6, Issue 3 (2025)
J Clinic Care Skill 2025, 6(3): 113-119 |
Back to browse issues page
Article Type:
Subject:
Ethics code: IR.SBMU.NNFTRI.REC.1399.067
History
Received: 2025/06/12 | Accepted: 2025/07/19 | Published: 2025/08/15
Received: 2025/06/12 | Accepted: 2025/07/19 | Published: 2025/08/15
How to cite this article
Haghshenosabet F, Eslamian G, Kazemi S, Rashidkhani B. Association Between Ultra-Processed Foods Before Pregnancy and Hyperemesis Gravidarum. J Clinic Care Skill 2025; 6 (3) :113-119
URL: http://jccs.yums.ac.ir/article-1-416-en.html
URL: http://jccs.yums.ac.ir/article-1-416-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- “Department of Cellular and Molecular Nutrition, Faculty of Nutrition and Food Technology” and “National Nutrition and Food Technology Research Institute”, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2- “Department of Obstetrics and Gynecology, Faculty of Medicine” and “Preventative Gynecology Research Center”, Shahid Beheshti University of Medical Sciences, Tehran, Iran
3- “Department of Community Nutrition, Faculty of Nutrition and Food Technology” and “National Nutrition and Food Technology Research Institute”, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2- “Department of Obstetrics and Gynecology, Faculty of Medicine” and “Preventative Gynecology Research Center”, Shahid Beheshti University of Medical Sciences, Tehran, Iran
3- “Department of Community Nutrition, Faculty of Nutrition and Food Technology” and “National Nutrition and Food Technology Research Institute”, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Full-Text (HTML) (226 Views)
Introduction
Hyperemesis gravidarum (HG) is a debilitating pregnancy complication with a multifactorial etiology, characterized by severe and persistent nausea and vomiting (NVP) [1]. While NVP affects 50-80% of pregnant women, HG is a more severe condition, occurring in 0.3-3% of pregnancies and often requiring hospitalization [2, 3]. However, some populations report significantly higher incidence rates [4]. The clinical consequences of HG are profound, including maternal weight loss, dehydration, electrolyte imbalances (such as hypokalemia and metabolic alkalosis), nutritional deficiencies, and psychological distress [5]. Fetal complications may also arise, including low birth weight, preterm delivery, small-for-gestational-age infants, and even stillbirth [6]. Additionally, maternal risks such as preeclampsia and placental abruption have been associated with HG [7].
The exact pathogenesis of HG remains unclear, but several risk factors have been identified. These include young maternal age, nulliparity, multiple pregnancies, female fetus, family history of HG, prior HG in previous pregnancies, gestational trophoblastic disease, low pre-pregnancy BMI, obesity, Helicobacter pylori infection, hyperthyroidism, oxidative stress, and psychological factors [5]. Beyond these, pre-pregnancy lifestyle factors—such as vitamin deficiencies, alcohol consumption, sedentary behavior, and high intake of saturated fats—may further increase susceptibility to HG [8].
Processed meats, a major dietary source of saturated fats, have been implicated in various adverse health outcomes [9]. More broadly, ultra-processed foods (UPFs), defined by extensive industrial processing, have seen a dramatic global increase in consumption over recent decades due to their convenience, long shelf life, and hyperpalatability [10, 11]. The NOVA classification system categorizes foods based on processing level, with UPFs typically being energy-dense and high in added sugars, salt, and unhealthy fats, while lacking fiber and essential micronutrients [12-14]. Numerous observational studies have linked UPF consumption to non-communicable diseases, including metabolic syndrome, cardiovascular disease, and certain cancers [15, 16].
Given that UPFs are a major source of saturated fats and may promote systemic inflammation and oxidative stress—both hypothesized mechanisms in HG pathogenesis—we sought to investigate their potential role. To date, no study has examined the association between UPF intake and HG risk. Therefore, this case-control study aimed to evaluate whether pre-pregnancy consumption of UPFs is associated with higher odds of developing HG in Iranian women, while adjusting for key confounding factors.
Instrument and Methods
Study design and sample
The present hospital-based case-control study was conducted between April 2020 and June 2021 in the obstetrics and gynecology departments of general hospitals across Tehran, Iran. The study population comprised 45 pregnant women diagnosed with HG and 135 healthy pregnant controls without HG. Case identification and diagnosis were performed according to the standardized criteria established by the American College of Obstetricians and Gynecologists (ACOG) [17].
The diagnostic criteria for HG were persistent vomiting unrelated to other medical conditions, biochemical evidence of acute starvation (ketonuria), and documented weight loss exceeding 5% of pre-pregnancy body weight.
For the HG case group, we established rigorous inclusion criteria to ensure the homogeneity of the study population. Eligible participants were women aged 18-35 years with singleton pregnancies at gestational ages below 14 weeks. Additional exclusion criteria included a history of alcohol consumption or substance use, utilization of assisted reproductive technologies, current Helicobacter pylori infection, adherence to specialized dietary regimens prior to pregnancy, the presence of chronic metabolic disorders (including diabetes mellitus and cardiovascular diseases), a history of swallowing disorders or eating pathologies, and current use of estrogen-containing medications.
The research was conducted in full compliance with the ethical principles outlined in the latest version of the Helsinki Declaration. Ethical approval was obtained from the Institutional Review Board of the National Nutrition and Food Technology Research Institute at Shahid Beheshti University of Medical Sciences in Tehran, Iran. Prior to participation, all enrolled subjects, including both HG cases and controls, provided written informed consent.
Control group participants were selected based on the absence of a current or previous HG diagnosis. For both cases and controls, we implemented additional exclusion criteria to ensure data quality, as reported daily energy intake values falling outside three standard deviations from the study population mean, and incomplete food frequency questionnaire responses, with more than 60 items left unanswered.
Instrument and measurements
Trained research personnel collected comprehensive demographic and clinical data through structured interviews. The collected information included maternal age, gestational week, parity, smoking status, family history of HG, socioeconomic indicators (monthly household income, educational attainment, and occupational status), and detailed medication/supplement use history.
Anthropometric measurements were obtained following standardized protocols. Height measurements were performed by a single trained examiner using a calibrated stadiometer, with participants standing barefoot in an erect position and their shoulders in neutral alignment; measurements were recorded to the nearest millimeter. Pre-pregnancy weight and waist circumference values were obtained through self-report. For participants who could not recall these measurements, we extracted the earliest available anthropometric data from prenatal medical records. Body mass index (BMI) was calculated using the standard formula: weight (kg) divided by height squared (m²). Physical activity levels were quantified using a validated questionnaire that has demonstrated reliability in previous studies [18].
Dietary intake data were collected by trained dietitians using a validated 168-item semi-quantitative food frequency questionnaire (FFQ) [19]. This interviewer-administered instrument, adapted from Willett’s FFQ [20], assessed habitual dietary patterns during the year preceding pregnancy. The FFQ incorporated standardized portion sizes for all food items. During administration, interviewers provided visual aids and detailed explanations of portion sizes before querying consumption frequencies (daily, weekly, monthly, or yearly).
Data collection
The research team converted reported food consumption frequencies into gram quantities using standardized household measures. For composite dishes (e.g., pizza), nutrient composition was determined based on typical restaurant preparation methods. Nutrient and energy values were calculated using both the USDA Food Composition Database and the Iranian Food Composition Table. UPFs were classified according to the NOVA food classification system [21], with 37 FFQ items aggregated into seven distinct categories. Daily UPF intake was expressed in grams per day.
Following rigorous data quality control measures, we excluded participants with extreme energy intake values, defined as log-transformed total energy intake exceeding ±3 standard deviations from the mean. This resulted in the exclusion of 3 HG cases and 5 control participants. Additionally, we excluded 2 HG cases and 3 controls who had incomplete dietary data, having skipped 60 or more items on the FFQ. These exclusions yielded final participation rates of 90% among HG cases and 93% among controls, with 45 cases and 126 controls included in the final analysis.
Statistical analysis
All statistical analyses were performed using SPSS 20. We employed two-tailed hypothesis tests throughout the study, with a predetermined significance level of α=0.05. The normality of continuous data was assessed through multiple approaches, including visual inspection of histograms and Q-Q plots, supplemented by a formal Kolmogorov-Smirnov test. Group comparisons were performed using the Mann-Whitney U test for continuous parameters and the chi-square test for categorical parameters.
UPF intake values were categorized into tertiles based on the distribution observed in the control group. We employed multiple binary logistic regression analyses to examine the association between UPF intake and HG risk. Two progressively adjusted models were constructed; Model 1 was adjusted for age and UPF-derived energy intake, and Model 2 was additionally adjusted for gestational week, family history of HG, previous HG episodes, BMI, total fiber intake, and physical activity level (in MET-hours/day). Trend analyses were conducted by treating the categorical tertile parameters as continuous predictors in the logistic regression models. All regression results are presented as odds ratios (OR) with corresponding 95% confidence intervals (CI).
Findings
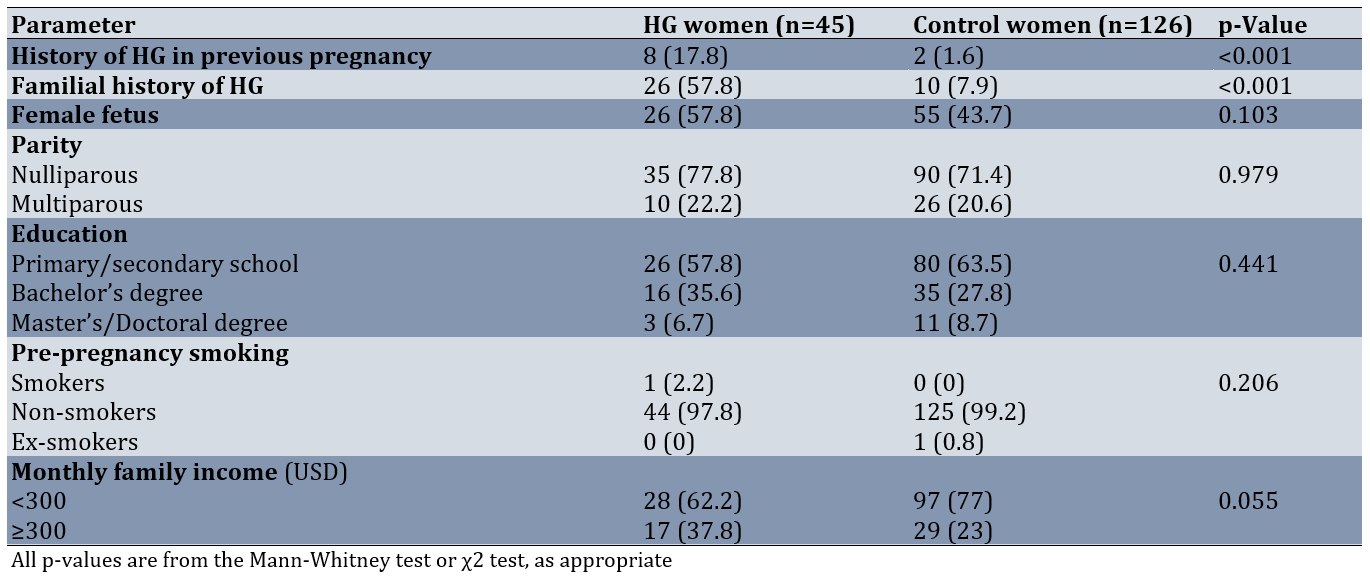
The mean age of participants was comparable between groups, with HG cases averaging 25.3±3.3 years and controls averaging 25.5±3.6 years, confirming the successful implementation of our frequency-matching design. The median age for women with hyperemesis gravidarum was 25 years (interquartile range (IQR): 23-27), while for control women, it was also 25 years (IQR: 23-28), with no significant difference between the groups (p=0.679). The median body mass index (BMI) for women with HG was 22.7kg/m² (IQR: 21.7-25.9), while for control women, it was 22.9kg/m² (IQR: 21.4-25), with no significant difference between the groups (p=0.284). Pre-pregnancy physical activity (metabolic equivalents per hour per day) was 28.2 (16.5-85.1) in women with HG and 45.1 (22.7-69.2) in control women (p=0.697). The median pregnancy week for women with HG was 11 (IQR: 10-12), while for control women, it was also 11 (IQR: 9-12; p=0.154). The median waist circumference for women with HG was 82cm (IQR: 80-84), while for control women, it was 80 cm (IQR: 79-83.6), with no significant difference between the groups (p=0.202). Notably, we observed significantly higher rates of both familial HG history (p<0.05) and previous pregnancy HG episodes (p<0.05) among cases compared to controls. However, the groups showed no significant differences in gestational week, parity, fetal sex distribution, pre-pregnancy anthropometric measurements (including BMI and waist circumference), educational attainment, employment status, pre-pregnancy physical activity levels, or smoking history (Table 1).
Table 1. Comparison of general characteristics of participants with hyperemesis gravidarum (HG) and controls
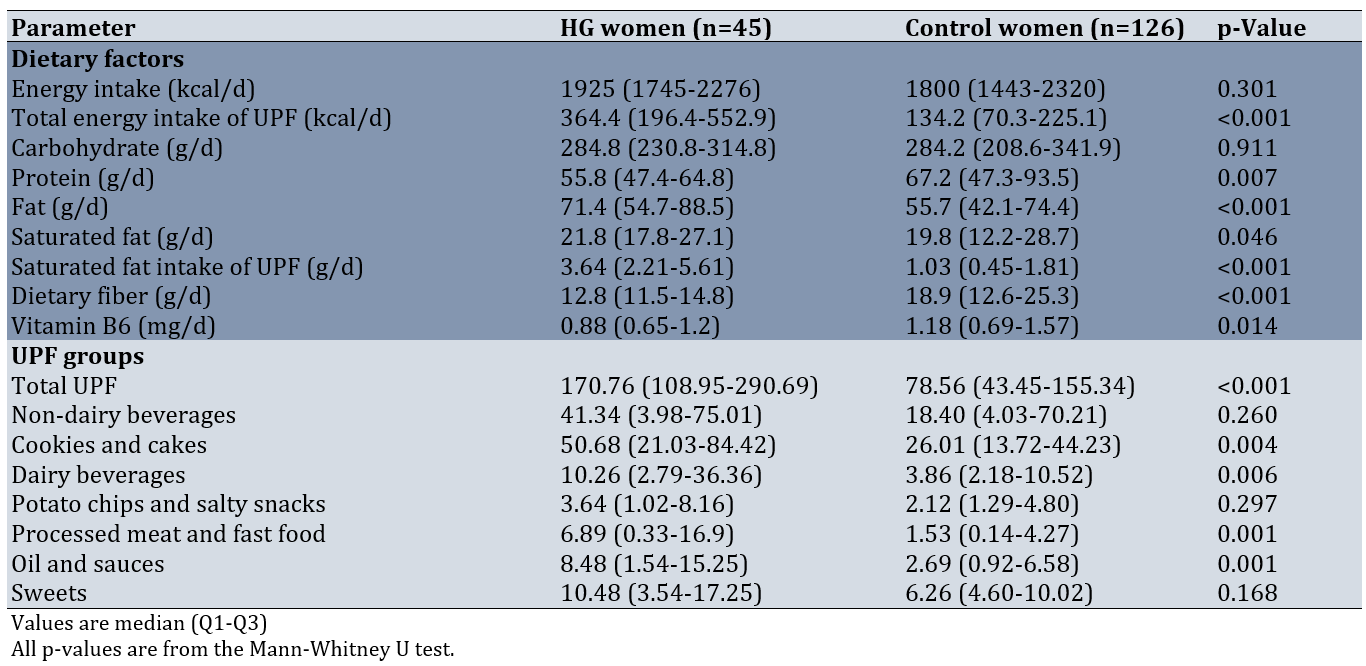
There were significant differences in median consumption levels between groups across multiple dietary components. HG cases demonstrated significantly higher intake of several UPF categories, including total energy derived from UPFs (p<0.01), total fat (p<0.05), saturated fat (p<0.05), cookies and cakes (p<0.05), processed meats (p<0.01), dairy beverages (p<0.05), fast foods (p<0.01), and oils and sauces (p<0.05). Conversely, control participants showed significantly higher consumption of dietary fiber (p<0.01), vitamin B6 (p<0.05), and total protein (p<0.05) compared to HG cases (Table 2).
Table 2. Comparison of energy, nutrient intakes, and dietary UPF intakes among hyperemesis gravidarum (HG) cases and controls
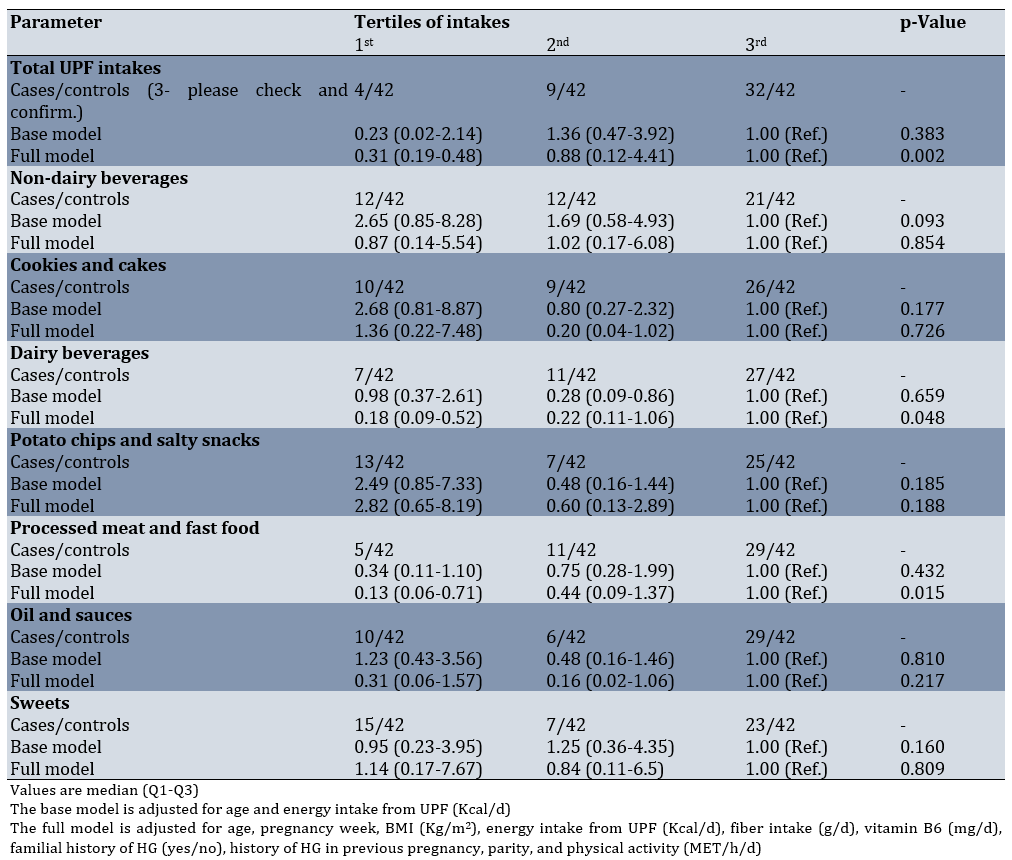
In fully adjusted models, we observed significant inverse associations between UPF intake and HG risk. Comparing the highest to lowest tertiles of consumption: total UPF intake showed an OR of 0.31 (95% CI: 0.19-0.48; p-value=0.002); dairy beverages showed an OR of 0.18 (95% CI: 0.09-0.52; p-value =0.048); and processed meats and fast foods showed an OR of 0.13 (95% CI: 0.06-0.71; p- value=0.015). These associations demonstrated consistent and statistically significant dose-response trends. Importantly, we found no significant associations between HG risk and the consumption of non-dairy beverages, cookies and cakes, potato chips and salty snacks, oils and sauces, or sweets in either crude or adjusted regression models (Table 3).
Table 3. Adjusted odds ratio (OR) estimates and 95% confidence intervals (CIs) for hyperemesis gravidarum (HG) according to the tertile of ultra-processed food (UPF) groups using a logistic regression model
Discussion
This study aimed to evaluate the association between pre-pregnancy consumption of UPFs and higher odds of developing HG in Iranian women. Our analysis revealed a significant association between UPF intake and HG odds after adjusting for potential confounders. While certain UPF categories demonstrated protective associations with HG risk, the overall dietary pattern characterized by higher UPF consumption appeared to be associated with increased risk, particularly when considering the parallel findings of reduced fiber and nutrient intake among HG cases. The differential effects observed across UPF subcategories emphasize the importance of examining both food processing methods and nutrient composition when investigating dietary risk factors for HG [22]. This study represents the first investigation of the UPF-HG association in a developing nation, building upon previous work that identified seafood, water, and vegetables as potential protective dietary factors [22].
HG remains a serious pregnancy complication that frequently necessitates hospitalization during the first trimester, underscoring the importance of identifying preventive strategies [3]. Our findings support the growing evidence that nutritional status plays a critical role in HG development [22-24]. The utilization of UPF as a marker of dietary quality has become increasingly valuable in nutritional epidemiology for examining diet-disease relationships [12]. Participants in the lowest tertile of total UPF consumption showed 69% lower odds of HG compared to those in the highest tertile. Notably, higher intakes of dairy beverages and processed meats/fast foods were also associated with reduced HG risk, suggesting complex relationships that warrant further investigation.
The mechanisms linking UPF consumption with HG risk may involve several pathways. While the exact etiology of HG remains unclear, existing evidence indicates that systemic inflammation markers are elevated in HG patients [25]. Diets with higher proportions of UPFs exhibit greater pro-inflammatory potential [26], with one study specifically showing that increased industrial food processing correlates with higher dietary inflammatory index scores in pregnant women [26]. Such pro-inflammatory diets may promote oxidative stress and enhance lipid peroxidation, processes that have been implicated in various pregnancy complications, including HG [27, 28]. The well-documented imbalance between pro-oxidants and antioxidants in HG pathogenesis [27] may be exacerbated by UPF consumption, as these products typically contain high levels of saturated fats and pro-oxidant components while being deficient in antioxidant compounds [12]. Additionally, industrial processing methods, including mechanical and thermal treatments, may further compromise the antioxidant properties of foods [29], potentially contributing to the observed associations.
Our findings regarding specific UPF categories provide important insights. The dairy beverages (including various ice creams and flavored milks) and processed meats/fast foods (such as burgers, sausages, and pizza) were characteristically high in total and saturated fats while being relatively low in antioxidant density. These nutritional characteristics may help explain their distinct associations with HG risk. Given the widespread consumption of UPFs globally, pregnant women may be particularly vulnerable to the potential adverse effects of processing-related ingredients and nutrient imbalances.
The current study possesses several notable strengths, including high participation rates and rigorous methodology to minimize information bias. All dietary assessments were conducted by trained dietitians who were blinded to participant case status, and we controlled for numerous potential confounding factors in our analyses. However, certain limitations must be acknowledged. The case-control design carries inherent risks of recall and selection bias, although we mitigated this concern by focusing on newly diagnosed HG cases. Our sample size, while adequate for initial exploration, became relatively small when divided into tertiles of UPF consumption, potentially limiting statistical power. Although we employed a validated FFQ that has demonstrated reliability in Iranian populations, some degree of dietary misclassification is inevitable in nutritional epidemiology. Additionally, cultural considerations precluded the assessment of alcohol consumption, which may represent a potential confounder.
In summary, pre-pregnancy dietary patterns characterized by lower consumption of UPFs, particularly dairy beverages and processed meats/fast foods, are associated with reduced odds of developing HG in Iranian women. These results suggest that limiting processed foods in the pre-conception period may play an important role in HG prevention. Healthcare providers, particularly dietitians working with women of reproductive age, should consider these findings when providing dietary counseling. Future research should employ larger longitudinal designs and examine comprehensive dietary patterns rather than isolated nutrients or food groups to better elucidate the mechanisms linking pre-pregnancy diet with HG risk. Such investigations would significantly advance our understanding of this challenging condition and inform evidence-based preventive strategies.
Conclusion
Lower pre-pregnancy ultra-processed food consumption is associated with reduced odds of developing HG.
Acknowledgments: The authors thank all the volunteers who participated in the study.
Ethical Permissions: The study was approved by the ethics committee of the National Nutrition and Food Technology Research Institute, Shahid Beheshti University of Medical Sciences, Tehran, Iran (IR.SBMU.NNFTRI.REC.1399.067).
Conflicts of Interests: None of the authors had any conflicts of interest to report.
Authors' Contribution: Haghshenosabet F (First Author), Methodologist/Main Researcher/Discussion Writer (30%); Eslamian G (Second Author), Methodologist/Discussion Writer/Statistical Analyst (30%); Kazemi SN (Third Author), Assistant Researcher (20%); Rashidkhani B (Fourth Author), Introduction Writer/Methodologist/Main or Assistant Researcher/Discussion Writer/Statistical Analyst (20%)
Funding/Support: We also appreciate the National Nutrition and Food Technology Research Institute at Shahid Beheshti University of Medical Sciences for their financial support of this study (Project NO. 00/26260).
Hyperemesis gravidarum (HG) is a debilitating pregnancy complication with a multifactorial etiology, characterized by severe and persistent nausea and vomiting (NVP) [1]. While NVP affects 50-80% of pregnant women, HG is a more severe condition, occurring in 0.3-3% of pregnancies and often requiring hospitalization [2, 3]. However, some populations report significantly higher incidence rates [4]. The clinical consequences of HG are profound, including maternal weight loss, dehydration, electrolyte imbalances (such as hypokalemia and metabolic alkalosis), nutritional deficiencies, and psychological distress [5]. Fetal complications may also arise, including low birth weight, preterm delivery, small-for-gestational-age infants, and even stillbirth [6]. Additionally, maternal risks such as preeclampsia and placental abruption have been associated with HG [7].
The exact pathogenesis of HG remains unclear, but several risk factors have been identified. These include young maternal age, nulliparity, multiple pregnancies, female fetus, family history of HG, prior HG in previous pregnancies, gestational trophoblastic disease, low pre-pregnancy BMI, obesity, Helicobacter pylori infection, hyperthyroidism, oxidative stress, and psychological factors [5]. Beyond these, pre-pregnancy lifestyle factors—such as vitamin deficiencies, alcohol consumption, sedentary behavior, and high intake of saturated fats—may further increase susceptibility to HG [8].
Processed meats, a major dietary source of saturated fats, have been implicated in various adverse health outcomes [9]. More broadly, ultra-processed foods (UPFs), defined by extensive industrial processing, have seen a dramatic global increase in consumption over recent decades due to their convenience, long shelf life, and hyperpalatability [10, 11]. The NOVA classification system categorizes foods based on processing level, with UPFs typically being energy-dense and high in added sugars, salt, and unhealthy fats, while lacking fiber and essential micronutrients [12-14]. Numerous observational studies have linked UPF consumption to non-communicable diseases, including metabolic syndrome, cardiovascular disease, and certain cancers [15, 16].
Given that UPFs are a major source of saturated fats and may promote systemic inflammation and oxidative stress—both hypothesized mechanisms in HG pathogenesis—we sought to investigate their potential role. To date, no study has examined the association between UPF intake and HG risk. Therefore, this case-control study aimed to evaluate whether pre-pregnancy consumption of UPFs is associated with higher odds of developing HG in Iranian women, while adjusting for key confounding factors.
Instrument and Methods
Study design and sample
The present hospital-based case-control study was conducted between April 2020 and June 2021 in the obstetrics and gynecology departments of general hospitals across Tehran, Iran. The study population comprised 45 pregnant women diagnosed with HG and 135 healthy pregnant controls without HG. Case identification and diagnosis were performed according to the standardized criteria established by the American College of Obstetricians and Gynecologists (ACOG) [17].
The diagnostic criteria for HG were persistent vomiting unrelated to other medical conditions, biochemical evidence of acute starvation (ketonuria), and documented weight loss exceeding 5% of pre-pregnancy body weight.
For the HG case group, we established rigorous inclusion criteria to ensure the homogeneity of the study population. Eligible participants were women aged 18-35 years with singleton pregnancies at gestational ages below 14 weeks. Additional exclusion criteria included a history of alcohol consumption or substance use, utilization of assisted reproductive technologies, current Helicobacter pylori infection, adherence to specialized dietary regimens prior to pregnancy, the presence of chronic metabolic disorders (including diabetes mellitus and cardiovascular diseases), a history of swallowing disorders or eating pathologies, and current use of estrogen-containing medications.
The research was conducted in full compliance with the ethical principles outlined in the latest version of the Helsinki Declaration. Ethical approval was obtained from the Institutional Review Board of the National Nutrition and Food Technology Research Institute at Shahid Beheshti University of Medical Sciences in Tehran, Iran. Prior to participation, all enrolled subjects, including both HG cases and controls, provided written informed consent.
Control group participants were selected based on the absence of a current or previous HG diagnosis. For both cases and controls, we implemented additional exclusion criteria to ensure data quality, as reported daily energy intake values falling outside three standard deviations from the study population mean, and incomplete food frequency questionnaire responses, with more than 60 items left unanswered.
Instrument and measurements
Trained research personnel collected comprehensive demographic and clinical data through structured interviews. The collected information included maternal age, gestational week, parity, smoking status, family history of HG, socioeconomic indicators (monthly household income, educational attainment, and occupational status), and detailed medication/supplement use history.
Anthropometric measurements were obtained following standardized protocols. Height measurements were performed by a single trained examiner using a calibrated stadiometer, with participants standing barefoot in an erect position and their shoulders in neutral alignment; measurements were recorded to the nearest millimeter. Pre-pregnancy weight and waist circumference values were obtained through self-report. For participants who could not recall these measurements, we extracted the earliest available anthropometric data from prenatal medical records. Body mass index (BMI) was calculated using the standard formula: weight (kg) divided by height squared (m²). Physical activity levels were quantified using a validated questionnaire that has demonstrated reliability in previous studies [18].
Dietary intake data were collected by trained dietitians using a validated 168-item semi-quantitative food frequency questionnaire (FFQ) [19]. This interviewer-administered instrument, adapted from Willett’s FFQ [20], assessed habitual dietary patterns during the year preceding pregnancy. The FFQ incorporated standardized portion sizes for all food items. During administration, interviewers provided visual aids and detailed explanations of portion sizes before querying consumption frequencies (daily, weekly, monthly, or yearly).
Data collection
The research team converted reported food consumption frequencies into gram quantities using standardized household measures. For composite dishes (e.g., pizza), nutrient composition was determined based on typical restaurant preparation methods. Nutrient and energy values were calculated using both the USDA Food Composition Database and the Iranian Food Composition Table. UPFs were classified according to the NOVA food classification system [21], with 37 FFQ items aggregated into seven distinct categories. Daily UPF intake was expressed in grams per day.
Following rigorous data quality control measures, we excluded participants with extreme energy intake values, defined as log-transformed total energy intake exceeding ±3 standard deviations from the mean. This resulted in the exclusion of 3 HG cases and 5 control participants. Additionally, we excluded 2 HG cases and 3 controls who had incomplete dietary data, having skipped 60 or more items on the FFQ. These exclusions yielded final participation rates of 90% among HG cases and 93% among controls, with 45 cases and 126 controls included in the final analysis.
Statistical analysis
All statistical analyses were performed using SPSS 20. We employed two-tailed hypothesis tests throughout the study, with a predetermined significance level of α=0.05. The normality of continuous data was assessed through multiple approaches, including visual inspection of histograms and Q-Q plots, supplemented by a formal Kolmogorov-Smirnov test. Group comparisons were performed using the Mann-Whitney U test for continuous parameters and the chi-square test for categorical parameters.
UPF intake values were categorized into tertiles based on the distribution observed in the control group. We employed multiple binary logistic regression analyses to examine the association between UPF intake and HG risk. Two progressively adjusted models were constructed; Model 1 was adjusted for age and UPF-derived energy intake, and Model 2 was additionally adjusted for gestational week, family history of HG, previous HG episodes, BMI, total fiber intake, and physical activity level (in MET-hours/day). Trend analyses were conducted by treating the categorical tertile parameters as continuous predictors in the logistic regression models. All regression results are presented as odds ratios (OR) with corresponding 95% confidence intervals (CI).
Findings
The mean age of participants was comparable between groups, with HG cases averaging 25.3±3.3 years and controls averaging 25.5±3.6 years, confirming the successful implementation of our frequency-matching design. The median age for women with hyperemesis gravidarum was 25 years (interquartile range (IQR): 23-27), while for control women, it was also 25 years (IQR: 23-28), with no significant difference between the groups (p=0.679). The median body mass index (BMI) for women with HG was 22.7kg/m² (IQR: 21.7-25.9), while for control women, it was 22.9kg/m² (IQR: 21.4-25), with no significant difference between the groups (p=0.284). Pre-pregnancy physical activity (metabolic equivalents per hour per day) was 28.2 (16.5-85.1) in women with HG and 45.1 (22.7-69.2) in control women (p=0.697). The median pregnancy week for women with HG was 11 (IQR: 10-12), while for control women, it was also 11 (IQR: 9-12; p=0.154). The median waist circumference for women with HG was 82cm (IQR: 80-84), while for control women, it was 80 cm (IQR: 79-83.6), with no significant difference between the groups (p=0.202). Notably, we observed significantly higher rates of both familial HG history (p<0.05) and previous pregnancy HG episodes (p<0.05) among cases compared to controls. However, the groups showed no significant differences in gestational week, parity, fetal sex distribution, pre-pregnancy anthropometric measurements (including BMI and waist circumference), educational attainment, employment status, pre-pregnancy physical activity levels, or smoking history (Table 1).
Table 1. Comparison of general characteristics of participants with hyperemesis gravidarum (HG) and controls

There were significant differences in median consumption levels between groups across multiple dietary components. HG cases demonstrated significantly higher intake of several UPF categories, including total energy derived from UPFs (p<0.01), total fat (p<0.05), saturated fat (p<0.05), cookies and cakes (p<0.05), processed meats (p<0.01), dairy beverages (p<0.05), fast foods (p<0.01), and oils and sauces (p<0.05). Conversely, control participants showed significantly higher consumption of dietary fiber (p<0.01), vitamin B6 (p<0.05), and total protein (p<0.05) compared to HG cases (Table 2).
Table 2. Comparison of energy, nutrient intakes, and dietary UPF intakes among hyperemesis gravidarum (HG) cases and controls

In fully adjusted models, we observed significant inverse associations between UPF intake and HG risk. Comparing the highest to lowest tertiles of consumption: total UPF intake showed an OR of 0.31 (95% CI: 0.19-0.48; p-value=0.002); dairy beverages showed an OR of 0.18 (95% CI: 0.09-0.52; p-value =0.048); and processed meats and fast foods showed an OR of 0.13 (95% CI: 0.06-0.71; p- value=0.015). These associations demonstrated consistent and statistically significant dose-response trends. Importantly, we found no significant associations between HG risk and the consumption of non-dairy beverages, cookies and cakes, potato chips and salty snacks, oils and sauces, or sweets in either crude or adjusted regression models (Table 3).
Table 3. Adjusted odds ratio (OR) estimates and 95% confidence intervals (CIs) for hyperemesis gravidarum (HG) according to the tertile of ultra-processed food (UPF) groups using a logistic regression model

Discussion
This study aimed to evaluate the association between pre-pregnancy consumption of UPFs and higher odds of developing HG in Iranian women. Our analysis revealed a significant association between UPF intake and HG odds after adjusting for potential confounders. While certain UPF categories demonstrated protective associations with HG risk, the overall dietary pattern characterized by higher UPF consumption appeared to be associated with increased risk, particularly when considering the parallel findings of reduced fiber and nutrient intake among HG cases. The differential effects observed across UPF subcategories emphasize the importance of examining both food processing methods and nutrient composition when investigating dietary risk factors for HG [22]. This study represents the first investigation of the UPF-HG association in a developing nation, building upon previous work that identified seafood, water, and vegetables as potential protective dietary factors [22].
HG remains a serious pregnancy complication that frequently necessitates hospitalization during the first trimester, underscoring the importance of identifying preventive strategies [3]. Our findings support the growing evidence that nutritional status plays a critical role in HG development [22-24]. The utilization of UPF as a marker of dietary quality has become increasingly valuable in nutritional epidemiology for examining diet-disease relationships [12]. Participants in the lowest tertile of total UPF consumption showed 69% lower odds of HG compared to those in the highest tertile. Notably, higher intakes of dairy beverages and processed meats/fast foods were also associated with reduced HG risk, suggesting complex relationships that warrant further investigation.
The mechanisms linking UPF consumption with HG risk may involve several pathways. While the exact etiology of HG remains unclear, existing evidence indicates that systemic inflammation markers are elevated in HG patients [25]. Diets with higher proportions of UPFs exhibit greater pro-inflammatory potential [26], with one study specifically showing that increased industrial food processing correlates with higher dietary inflammatory index scores in pregnant women [26]. Such pro-inflammatory diets may promote oxidative stress and enhance lipid peroxidation, processes that have been implicated in various pregnancy complications, including HG [27, 28]. The well-documented imbalance between pro-oxidants and antioxidants in HG pathogenesis [27] may be exacerbated by UPF consumption, as these products typically contain high levels of saturated fats and pro-oxidant components while being deficient in antioxidant compounds [12]. Additionally, industrial processing methods, including mechanical and thermal treatments, may further compromise the antioxidant properties of foods [29], potentially contributing to the observed associations.
Our findings regarding specific UPF categories provide important insights. The dairy beverages (including various ice creams and flavored milks) and processed meats/fast foods (such as burgers, sausages, and pizza) were characteristically high in total and saturated fats while being relatively low in antioxidant density. These nutritional characteristics may help explain their distinct associations with HG risk. Given the widespread consumption of UPFs globally, pregnant women may be particularly vulnerable to the potential adverse effects of processing-related ingredients and nutrient imbalances.
The current study possesses several notable strengths, including high participation rates and rigorous methodology to minimize information bias. All dietary assessments were conducted by trained dietitians who were blinded to participant case status, and we controlled for numerous potential confounding factors in our analyses. However, certain limitations must be acknowledged. The case-control design carries inherent risks of recall and selection bias, although we mitigated this concern by focusing on newly diagnosed HG cases. Our sample size, while adequate for initial exploration, became relatively small when divided into tertiles of UPF consumption, potentially limiting statistical power. Although we employed a validated FFQ that has demonstrated reliability in Iranian populations, some degree of dietary misclassification is inevitable in nutritional epidemiology. Additionally, cultural considerations precluded the assessment of alcohol consumption, which may represent a potential confounder.
In summary, pre-pregnancy dietary patterns characterized by lower consumption of UPFs, particularly dairy beverages and processed meats/fast foods, are associated with reduced odds of developing HG in Iranian women. These results suggest that limiting processed foods in the pre-conception period may play an important role in HG prevention. Healthcare providers, particularly dietitians working with women of reproductive age, should consider these findings when providing dietary counseling. Future research should employ larger longitudinal designs and examine comprehensive dietary patterns rather than isolated nutrients or food groups to better elucidate the mechanisms linking pre-pregnancy diet with HG risk. Such investigations would significantly advance our understanding of this challenging condition and inform evidence-based preventive strategies.
Conclusion
Lower pre-pregnancy ultra-processed food consumption is associated with reduced odds of developing HG.
Acknowledgments: The authors thank all the volunteers who participated in the study.
Ethical Permissions: The study was approved by the ethics committee of the National Nutrition and Food Technology Research Institute, Shahid Beheshti University of Medical Sciences, Tehran, Iran (IR.SBMU.NNFTRI.REC.1399.067).
Conflicts of Interests: None of the authors had any conflicts of interest to report.
Authors' Contribution: Haghshenosabet F (First Author), Methodologist/Main Researcher/Discussion Writer (30%); Eslamian G (Second Author), Methodologist/Discussion Writer/Statistical Analyst (30%); Kazemi SN (Third Author), Assistant Researcher (20%); Rashidkhani B (Fourth Author), Introduction Writer/Methodologist/Main or Assistant Researcher/Discussion Writer/Statistical Analyst (20%)
Funding/Support: We also appreciate the National Nutrition and Food Technology Research Institute at Shahid Beheshti University of Medical Sciences for their financial support of this study (Project NO. 00/26260).
Keywords:
References
1. McCarthy FP, Lutomski JE, Greene RA. Hyperemesis gravidarum: Current perspectives. Int J Womens Health. 2014;6:719-25. [Link] [DOI:10.2147/IJWH.S37685]
2. Parker SE, Starr JR, Collett BR, Speltz ML, Werler MM. Nausea and vomiting during pregnancy and neurodevelopmental outcomes in offspring. Paediatr Perinat Epidemiol. 2014;28(6):527-35. [Link] [DOI:10.1111/ppe.12151]
3. Lacasse A, Rey E, Ferreira E, Morin C, Bérard A. Epidemiology of nausea and vomiting of pregnancy: Prevalence, severity, determinants, and the importance of race/ethnicity. BMC Pregnancy and Childbirth. 2009;9:26. [Link] [DOI:10.1186/1471-2393-9-26]
4. Fejzo MS, Ingles SA, Wilson M, Wang W, MacGibbon K, Romero R, et al. High prevalence of severe nausea and vomiting of pregnancy and hyperemesis gravidarum among relatives of affected individuals. Eur J Obstet Gynecol Reprod Biol. 2008;141(1):13-7. [Link] [DOI:10.1016/j.ejogrb.2008.07.003]
5. Kuşcu NK, Koyuncu F. Hyperemesis gravidarum: Current concepts and management. Postgrad Med J. 2002;78(916):76-9. [Link] [DOI:10.1136/pmj.78.916.76]
6. Sonkusare S. Hyperemesis gravidarum: A review. Med J Malaysia. 2008;63(3):272-6. [Link]
7. Bolin M, Åkerud H, Cnattingius S, Stephansson O, Wikström AK. Hyperemesis gravidarum and risks of placental dysfunction disorders: A population-based cohort study. BJOG. 2013;120(5):541-7. [Link] [DOI:10.1111/1471-0528.12132]
8. Kim HY, Cho GJ, Kim SY, Lee KM, Ahn KH, Han SW, et al. Pre-pregnancy risk factors for severe hyperemesis gravidarum: Korean population based cohort study. Life. 2020;11(1):12. [Link] [DOI:10.3390/life11010012]
9. O'Sullivan TA, Hafekost K, Mitrou F, Lawrence D. Food sources of saturated fat and the association with mortality: A meta-analysis. Am J Public Health. 2013;103(9):e31-42. [Link] [DOI:10.2105/AJPH.2013.301492]
10. Silva Meneguelli T, Viana Hinkelmann J, Hermsdorff HHM, Zulet MÁ, Martínez JA, Bressan J. Food consumption by degree of processing and cardiometabolic risk: A systematic review. Int J Food Sci Nutr. 2020;71(6):678-92. [Link] [DOI:10.1080/09637486.2020.1725961]
11. Weaver CM, Dwyer J, Fulgoni VL, King JC, Leveille GA, MacDonald RS, et al. Processed foods: Contributions to nutrition. Am J Clin Nutr. 2014;99(6):1525-42. [Link] [DOI:10.3945/ajcn.114.089284]
12. Elizabeth L, Machado P, Zinöcker M, Baker P, Lawrence M. Ultra-processed foods and health outcomes: A narrative review. Nutrients. 2020;12(7):1955. [Link] [DOI:10.3390/nu12071955]
13. Martínez Steele E, Popkin BM, Swinburn B, Monteiro CA. The share of ultra-processed foods and the overall nutritional quality of diets in the US: Evidence from a nationally representative cross-sectional study. Popul Health Metr. 2017;15(1):6. [Link] [DOI:10.1186/s12963-017-0119-3]
14. Monteiro CA, Cannon G, Levy R, Moubarac JC, Jaime P, Martins AP, et al. NOVA. The star shines bright. World Nutr. 2016;7(1-3):28-38. [Link]
15. Lawrence MA, Baker PI. Ultra-processed food and adverse health outcomes. BMJ 2019;365:l2289. [Link] [DOI:10.1136/bmj.l2289]
16. Poti JM, Braga B, Qin B. Ultra-processed food intake and obesity: What really matters for health-processing or nutrient content?. Curr Obes Rep. 2017;6(4):420-31. [Link] [DOI:10.1007/s13679-017-0285-4]
17. Committee on Practice Bulletins-Obstetrics. ACOG practice bulletin No. 189: Nausea and vomiting of pregnancy. Obstet Gynecol. 2018;131(1):e15-30. [Link] [DOI:10.1097/AOG.0000000000002456]
18. Aadahl M, Jørgensen T. Validation of a new self-report instrument for measuring physical activity. Med Sci Sports Exerc. 2003;35(7):1196-202. [Link] [DOI:10.1249/01.MSS.0000074446.02192.14]
19. Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. 2010;13(5):654-62. [Link] [DOI:10.1017/S1368980009991698]
20. Willett W, Hu F. The food frequency questionnaire. Cancer Epidemiol Biomarkers Prev. 2007;16(1):182-3. [Link] [DOI:10.1158/1055-9965.EPI-06-0843]
21. Edalati S, Bagherzadeh F, Asghari Jafarabadi M, Ebrahimi-Mamaghani M. Higher ultra-processed food intake is associated with higher DNA damage in healthy adolescents. Br J Nutr. 2021;125(5):568-76. [Link] [DOI:10.1017/S0007114520001981]
22. Haugen M, Vikanes A, Brantsaeter AL, Meltzer HM, Grjibovski AM, Magnus P. Diet before pregnancy and the risk of hyperemesis gravidarum. Br J Nutr. 2011;106(4):596-602. [Link] [DOI:10.1017/S0007114511000675]
23. Signorello LB, Harlow BL, Wang S, Erick MA. Saturated fat intake and the risk of severe hyperemesis gravidarum. Epidemiology. 1998;9(6):636-40. [Link] [DOI:10.1097/00001648-199811000-00013]
24. Haghshenosabet F, Eslamian G, Kazemi S, Rashidkhani B. Association between pre-pregnancy food group intakes and hyperemesis gravidarum: A case-control study. Iran J Obstet Gynecol Infertil. 2022;24(12):77-87. [Persian] [Link]
25. Kan E, Emektar E, Corbacioglu K, Safak T, Sariaydin T, Cevik Y. Evaluation of relationship between inflammatory markers and hyperemesis gravidarum in patients admitted to emergency department. Am J Emerg Med. 2020;38(2):292-5. [Link] [DOI:10.1016/j.ajem.2019.05.007]
26. Silva CA, Santos IDS, Shivappa N, Hebert JR, Crivellenti LC, Sartorelli DS. The role of food processing in the inflammatory potential of diet during pregnancy. Rev Saude Publica. 2019;53:113. [Link] [DOI:10.11606/S1518-8787.2019053001154]
27. Fait V, Sela S, Ophir E, Khoury S, Nissimov J, Tkach M, et al. Hyperemesis gravidarum is associated with oxidative stress. Am J Perinatol. 2002;19(2):93-8. [Link] [DOI:10.1055/s-2002-23554]
28. Duhig K, Chappell LC, Shennan AH. Oxidative stress in pregnancy and reproduction. Obstet Med. 2016;9(3):113-6. [Link] [DOI:10.1177/1753495X16648495]
29. Al-Juhaimi F, Ghafoor K, Özcan MM, Jahurul MHA, Babiker EE, Jinap S, et al. Effect of various food processing and handling methods on preservation of natural antioxidants in fruits and vegetables. J Food Sci Technol. 2018;55(10):3872-80. [Link] [DOI:10.1007/s13197-018-3370-0]










