Volume 5, Issue 1 (2024)
J Clinic Care Skill 2024, 5(1): 41-48 |
Back to browse issues page
Article Type:
Ethics code: IR.ZUMS.REC.1398.208
History
Received: 2023/12/18 | Accepted: 2024/03/1 | Published: 2024/03/12
Received: 2023/12/18 | Accepted: 2024/03/1 | Published: 2024/03/12
How to cite this article
Zakerian Zadeh A, Dadashi M, Maghbooli M, Zarei F, Zakeryanzade R. Assessment of Depression Symptoms, Motor Learning, and Cognitive Function after Transcranial Direct Current Stimulation in Ischemic Stroke. J Clinic Care Skill 2024; 5 (1) :41-48
URL: http://jccs.yums.ac.ir/article-1-227-en.html
URL: http://jccs.yums.ac.ir/article-1-227-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Clinical Psychology, Faculty of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran
2- “Department of Neurology, School of Medicine” and “Vali-e-Asr Hospital”, Zanjan University of Medical Sciences, Zanjan, Iran
3- Department of Psychology and Educational Sciences, Faculty of Humanities, Yasuj Branch, Islamic Azad University, Yasuj, Iran
2- “Department of Neurology, School of Medicine” and “Vali-e-Asr Hospital”, Zanjan University of Medical Sciences, Zanjan, Iran
3- Department of Psychology and Educational Sciences, Faculty of Humanities, Yasuj Branch, Islamic Azad University, Yasuj, Iran
Full-Text (HTML) (396 Views)
Introduction
Stroke is increasingly prevalent in developing countries and is the second leading cause of death worldwide and the primary cause of disability [1]. Symptoms of stroke include various types of impairments such as motor, sensory, linguistic, and other cognitive functions [2]. Motor impairments following stroke are very common and are major contributors to disability post-stroke, also providing a basis for persistent neurobiological and cognitive sequelae [3].
Approximately 70-80% of stroke patients suffer from motor deficits, and a significant portion of them experience cognitive impairments related to stroke. Motor and cognitive deficits in stroke are interrelated with strong associations between activities such as walking, balance, attentional cognitive domains, and executive functioning [4]. About two-thirds of stroke patients experience cognitive impairments in the acute phase; about 30% of these patients recover spontaneously, and many are still at risk for cognitive impairment [5, 6]. According to recent research, post-stroke cognitive impairment in adults may result in white matter damage in brain regions distant from the primary lesion site. Changes in cognitive function over time after stroke and their association with the trajectory of white matter integrity in the brain provide a pathway for patient management and identification in the early stages [7].
Despite the favorable improvement in physical symptoms, 50% of stroke patients still suffer from cognitive impairment or limitations in rebuilding their normal lives, and 30% exhibit symptoms of depression 2 to 3 years after the stroke [8]. The prevalence of depression has been reported in 10% to 60% of studies. Depression is one of the most common psychological consequences of stroke, often accompanied by persistent disability, cognitive impairments, and mortality. Its onset typically occurs 6 to 24 months after the stroke, which may last up to 3 years in some patients [9]. Given the significant prevalence of stroke and the likelihood of depression in a large percentage of affected patients, efforts to improve and treat depression not only reduce the risk of subsequent strokes but also improve functionality, reduce mortality, and decrease the need for post-stroke healthcare [10]. The determinants of depression after stroke largely depend on the location and extent of the lesion, silent infarction, white matter damage, and brain atrophy [11]. However, no association has been observed between cerebral vascular lesions and depression indicative of functional brain disturbances due to structural brain damage [12]. Treatments mainly focus on rehabilitating motor deficits, as motor impairments are more unpleasant than cognitive disabilities [13].
Transcranial Direct Current Stimulation (tDCS) is a non-invasive and painless technique that induces cortical excitability [14]. Based on the evidence, tDCS is considered a safe method in human and animal studies and has no known irreversible damage [15]. Depression symptoms are usually associated with changes in cortical activity and excitability, especially in prefrontal regions [16]. They reflect an alteration in a distributed cortico-subcortical network across the hemispheres [17]. Therefore, it is reasonable to assume that modifying this pathological state with brain stimulation techniques may provide a therapeutic target. In addition to repetitive transcranial magnetic stimulation, transcranial direct current stimulation (tDCS) modulates cortical excitability for hours after stimulation cessation, making it a promising non-invasive therapeutic option [18]. Stimulation of the dorsolateral prefrontal cortex (DLPFC) is the most common site of brain cortex stimulation for reducing depression symptoms and improving mood and cognitive and attentional functions [19].
When standard anodal tDCS is used for stimulation, cortical function improves compared to baseline, while cathodal stimulation inhibits motor potentials, thereby implicitly enhancing motor learning processes [20]. Researchers believe that left dorsolateral prefrontal cortex (DLPFC) stimulation accounts for 23% of the changes in upper limb motor function, along with improvements in executive function, working memory, and attentional performance [21], suggesting that cognitive domains should not be evaluated independently as they influence each other, much like motor functions impacting attentional capacities [5].
Recent studies indicate that tDCS enhances motor functions in chronic stroke patients [22, 23]. Facilitation of motor learning through cortical excitability modulation induced by anodal tDCS may strengthen neuronal connectivity, thus improving motor learning processes [22]. Kang et al. confirm the long-term effects of motor learning in stroke patients treated with tDCS [24]. Yamaguchi et al. demonstrate that a combination of attention and tDCS is an effective strategy for enhancing rehabilitation in stroke patients with neurologic impairments [25]. The left DLPFC region integrates retained information in active memory with upcoming actions, where the left DLPFC is involved in encoding information. At the same time, the right DLPFC is engaged in information retrieval [26]. Although the increase in activity in the DLPFC during planning a complex task aligns with the idea that the DLPFC links information (a visual cue) to future action (required movement), the impact of tDCS on the DLPFC has only been investigated in cognitive tasks such as active memory and executive function [27]. At the same time, its effect on motor behavior remains largely unknown [28].
Although non-invasive stimulation techniques like tDCS are recognized as potential, innovative, cost-effective, portable, and easily manageable therapeutic methods for improving both motor and cognitive abilities, there are still unknown factors. Customized protocols involving consecutive stimulation and inhibition of the motor cortex (M1 and M2) and stimulation and inhibition of the left and right DLPFC hemispheres have not yet been utilized in studies, and sufficient data on stimulating two brain regions simultaneously are lacking. Therefore, given the high prevalence of stroke-related complications such as motor and cognitive impairments and depression in ischemic stroke patients, the present study aimed to determine the effect of transcranial direct current stimulation (tDCS) on depression symptoms, motor learning, and cognitive function in ischemic stroke patients.
Materials and Methods
This randomized controlled clinical trial by transcranial direct current stimulation (tDCS) was conducted on all patients with ischemic stroke referred to Vali-Asr University Hospital in Zanjan City, Iran, during 2019-2020. According to Delavar, in experimental research, a minimum sample size of 15 individuals per group is required under controlled conditions [29]. A sample size of 15 individuals per group was considered. 70 patients were initially introduced to participate. Due to the COVID-19 pandemic, only 35 patients between 18 and 85 with a diagnosis of chronic ischemic stroke confirmed by imaging, basic education, right-hand, at least 3 months elapsed since the onset of the infarction, presence of a deficit based on measurement tools, and mild to severe depressive symptoms based on the Hamilton Depression Rating Scalemet were enrolled in the study. Individuals with suicidal ideation or risks that prevent maintaining drug dosage, psychiatric disorders, substance abuse, history of head injury and epileptic seizures, contraindications to tDCS use, seizures, and presence of a cardiac pacemaker were not entered. They were randomly allocated to three groups (11 patients in the tDCS group, 12 patients in the Sham group, and 12 patients in the control group) using the Random Number Generator v1.3 web-based program. A total of 11 patients withdrew from the study due to various reasons, such as unwillingness to undergo an MRI for diagnosis before the study, fear of the COVID-19 pandemic, consecutive absences from two intervention sessions, difficulty in mobility due to severe motor impairment, etc. Eventually, 24 patients with ischemic stroke remained (Figure 1).

Figure 1. Consort diagram of participants
Data Collection
The Fugl-Meyer test, Hamilton Depression Rating Scale, Montreal Cognitive Assessment (MCA), and Mini-Mental State Examination (MMSE) were used to assess motor learning, depression, and cognitive function, respectively.
The Fugl-Meyer Test was utilized to evaluate upper and lower extremity motor performance. The test items included 50 movements categorized into 6 levels of improvement, with 33 items related to the upper extremity in the shoulder, elbow, forearm, wrist, and hand. Each item is scored from 0 to 2. 0: if the individual is unable to perform the movement, 1: if the movement is incomplete, and 2: if the movement is fully performed. The total score for the upper extremity is 66 [30-32]. The reliability between the two examiners in this test in the study by Duncan & Stanford was 0.97 and 0.96, respectively [33]. In the study by Karimi et al., the reliability between two examiners was 0.977 [34].
The Hamilton Depression Rating Scale was used to measure depression in participants. This scale consists of 21 items. Higher scores indicate more severe depressive symptoms, with participant responses to questions classified as follows: 0-7 (no depression), 8-17 (mild depression), 18-24 (moderate depression), and above 25 (severe depression). The reliability and validity of this test have been reported as 0.90 [35]. The reliability (Cronbach's alpha) of this test was 0.88.
For cognitive performance evaluation, the Montreal Cognitive Assessment (MCA) and the Mini-Mental State Examination (MMSE) were used. The MCA is a cognitive screening questionnaire designed by Julayanont et al. [36] to detect mild cognitive impairment. The purpose of administering this test was to overcome the limitations of the MMSE (Mini-Mental State Examination). This test assesses 8 cognitive domains through various skills. The maximum score for this test is 30, and the cutoff point is set at 24. The test is completed in less than 15 minutes. For individuals with less than 12 years of education, 1 point is added to their total score. The reliability of this test, based on Cronbach's alpha, was 92%. In Iran, the Montreal Cognitive Assessment scale has been validated by Seigaroody et al. [37].
Procedure
After being registered on IRCT (Code 42561), and the definitive diagnosis of stroke by a neurology specialist and clinical psychology interview with the patient, the Fugl-Meyer Test for motor learning evaluation, the Hamilton Depression Rating Scale for depression measurement, and cognitive assessment tests including MoCA and MMSE for cognitive function assessment were done by the patient on paper. Additionally, brain imaging was performed with MRI to record the stroke status and diagnose the disease by a neurologist.
This study included three groups, and the intervention implementation for each group was as follows:
1- First group (real intervention group): Anodal stimulation was performed consecutively in the primary motor areas affected and the left dorsolateral prefrontal cortex (M1damH+Left-DLPFC), with 20 minutes of anodal stimulation to M1damH, followed by 30 minutes of rest without stimulation, and finally, 20 minutes of anodal stimulation to Left-DLPFC using the tDCS device. The tDCS intervention was conducted for a total of 12 sessions, one per day.
2- Second group (placebo intervention group): Anodal stimulation was performed in the primary motor areas affected and left dorsolateral prefrontal cortex (M1damH+Left-DLPFC). The anodal stimulation to M1damH was discontinued one minute after the start of stimulation, followed by 30 minutes of rest, and finally, anodal stimulation to Left-DLPFC, which was discontinued one minute after the start of stimulation. The stimulation was performed using the tDCS device.
3- Third group (control group): This group received only routine treatment.
In addition to the research interventions, the first and second groups also received routine treatment.
To assess the study's outcomes, questionnaires were completed after the sessions and one month later to evaluate motor learning, depressive symptoms, and cognitive function. The patients were unaware of the group they were assigned to and the type of intervention. Pre-test and post-test evaluations of the patients were performed by another individual other than the therapist.
Statistical analysis
The data were analyzed using descriptive statistics (mean, standard deviation, frequency, and tables) and inferential statistics (multivariate analysis of covariance) with the necessary assumptions using SPSS 23 software.
Findings
The mean age of the patients was 64.12±14.8 years (p=0.062), and the mean time since stroke was 47.60±38.99 months (p=0.437). There were no significant differences in demographic characteristics between the study groups (Table 1).
Table 1. Comparing the frequency of demographic characteristics between the study groups

The assumption of homogeneity of variances was met, and there was no obstacle to performing a multivariate analysis of covariance. Based on the multivariate analysis using Wilks’, tDCS and Sham interventions significantly affected the study groups regarding motor performance, depressive symptoms, and cognitive function, as measured by MMSE and MOCA (p<0.05). The T-test showed a significant effect of tDCS on post-test and follow-up motor performance, depressive symptoms, and MMSE, MoCA cognitive function in the tDCS intervention group (p<0.05). At the same time, it was not significant in the Sham and Control groups (Table 2).
Table 2. Wilks' Lambda multivariate test for research variables (df=4; err. df=38)

There was a significant difference between the study groups in motor learning, depression, and cognitive performance assessed at post-intervention and 1-month follow-up (p<0.001; Table 3). The effect size ranged from 30 to 63%, indicating a substantial impact of the intervention and improvement in post-intervention and follow-up assessments (Figure 2).
Table 3. Results of multivariate analysis of covariance between groups in post-test and follow-up (df=2)

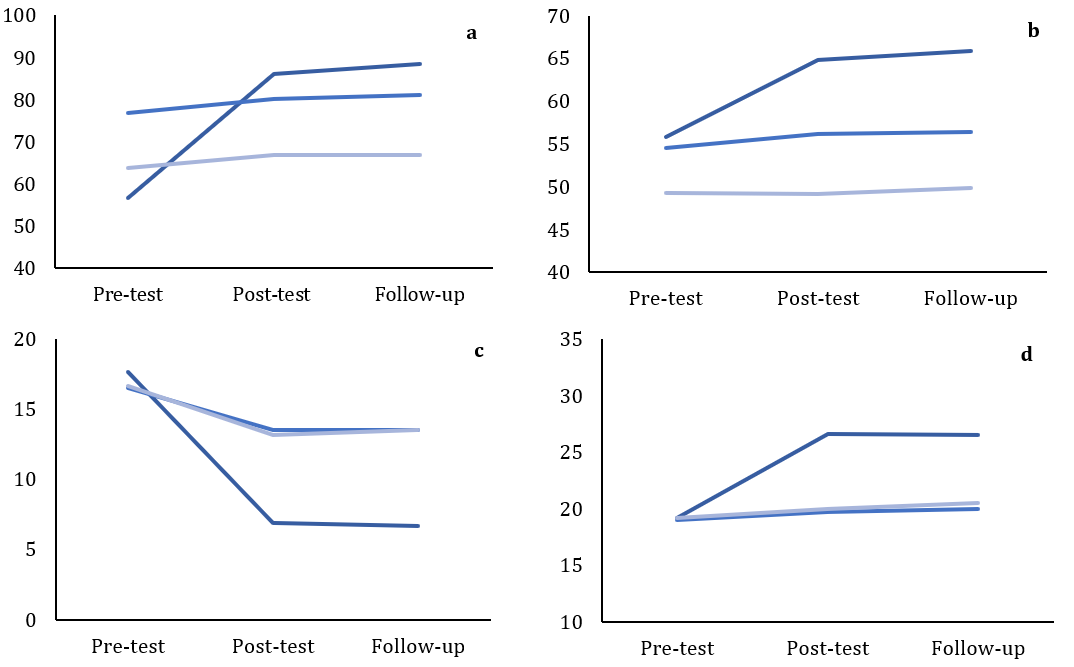

Figure 2. Comparing the score of (a) motor performance in the upper limbs, (b) motor performance in the lower limbs, (c) Hamilton depression, (d) Mini-Mental State Examination (MMSE), and (e) Montreal Cognitive Assessment (MCA)
There were significant differences in cognitive performance, depressive symptoms, and MoCA cognitive function between the tDCS and the Sham and Control groups in the post-test and follow-up (p<0.05). Similarly, there was no significant difference between the Sham and Control groups in cognitive performance, depressive symptoms, and MMSE and MoCA cognitive function in the post-intervention and follow-up assessments (Table 4).
Table 4. Comparing between tDCS and Sham and control (using the Bonferroni follow-up test) at post-intervention and follow-up

Discussion
This study aimed to evaluate depression symptoms, motor learning, and cognitive function after transcranial direct current stimulation (tDCS) in ischemic stroke patients. The novelty of this research is the simultaneous anodal stimulation of two areas. The results indicated that tDCS improved depression symptoms, motor function, and cognitive function in patients with stroke, while no significant improvement was observed in the sham and control groups.
In this study, tDCS improved motor learning. Similarly, using a systematic review and meta-analysis, Šimko et al. have shown that anodal stimulation in the damaged hemisphere and cathodal inhibition in the contralateral hemisphere led to long-term improvement in motor learning with tDCS [21]. Additionally, Yamaguchi et al. have found that tDCS enhances motor learning in healthy individuals by stimulating the brain cortex [25]. In accordance with this study, Elsner et al. have demonstrated that tDCS improves motor function in stroke patients [38, 39]. Oveisgharan et al. have reported that left DLPFC stimulation combined with M1 stimulation resulted in greater improvement in motor function compared to M1 stimulation alone, with left DLPFC stimulation being safe and effective in improving hand paralysis after acute stroke [40]. Furthermore, Elsner et al. have shown improvement in upper and lower limb function, muscle strength, and cognitive ability with tDCS after stroke, highlighting its importance [38].
The present study differs from previous ones in the number of sessions and the intervention protocol (sequential anodal stimulation of M1 and DLPFC in the affected hemisphere). Previous interventions focused on anodal stimulation in a single brain region (M1 or DLPFC), whereas this study compared three groups: Active stimulation, Sham, and control, which were not previously examined.
Kang et al. [24] stated that each of the three tDCS protocols (anodal stimulation on M1 in the affected hemisphere, cathodal stimulation on M1 in the unaffected hemisphere, and bilateral stimulation on both hemispheres) produces long-term learning effects, significantly improving stroke patients' recovery. It has been shown that brain stimulation and rehabilitation can increase upper limb improvement by 20% in the intervention group compared to the control, which were statistically significant [41]. This suggests that tDCS could be an effective intervention alongside other rehabilitation methods for improving movement in stroke patients [37, 38].
A-tDCS enhances cortical excitability under all conditions, immediately after stimulation; Therefore, the efficacy of A-tDCS depends on the current density and duration of use [25]. A-tDCS increases cortical excitability in both healthy individuals and stroke patients, favoring improved motor function following A-tDCS [42]. The neural circuits associated with ankle tracking during motor tasks are likely activated or enhanced, which may facilitate synaptic plasticity during tDCS, resulting in improvement [43]. Motor learning usually involves activity-dependent synaptic changes, leading to neural plasticity such as LTP-like or LTD-like changes in cortical neurons [43], which can sustain improvement after intervention cessation.
Cognitive function in stroke patients improved after tDCS intervention. Similarly, Berryhill & Martin have demonstrated that tDCS creates stable and long-term cognitive effects in clinical and healthy samples [44].
Furthermore, depression symptoms improved in patients after the tDCS intervention. Consistent with the present findings, Shiozawa et al. have shown that brain electrical stimulation can reduce depressive symptoms [45].
It can be suggested that the observed effects may result from increased cortical excitability in the left lateral prefrontal cortex, as anodal stimulation depolarizes neurons, leading to changes in neuronal resting state and excitability in that area [46]. This stimulation enhances the networks involved in various cognitive tasks. Another hypothesis could involve the role of dopamine in memory and cognitive tasks. Increased excitability in the superficial layers of the prefrontal cortex may lead to increased dopamine release, which in turn may improve cognitive performance and problem-solving. Additionally, constant electrical flow can lead to changes in local ion concentration, affecting membrane-permeable proteins and hydrogen ions (H+) [47], contributing to improved excitability. Another hypothesis could involve activating other brain regions by stimulating the lateral posterior prefrontal cortex, which may improve short-term memory, visuospatial skills, executive functions, attention, concentration, working memory, language, and awareness of time and place in stroke patients [48].
Another interpretation regarding the efficacy of tDCS on cognitive functions in the follow-up evaluation showed that the improvement in cognitive functions compared to the post-test decreased. Considering that cognitive functions are a complex and widespread capability throughout the brain, particularly involving the prefrontal areas, it seems that increasing the number of tDCS stimulation sessions or cyclically administering tDCS after the completion of the initial ten-session period may help to sustain the effects of cognitive function change and improvement over time.
To sum up, brain electrical stimulation is a suitable and cost-effective intervention for improving cognitive functions and motor learning, and reducing depressive symptoms after stroke can be beneficial. Therefore, for cognitive rehabilitation and prevention of disorders in healthcare and counseling centers, it is necessary to consider this type of intervention as a safe method, in addition to pharmacological treatment and other rehabilitation methods for stroke patients.
This study had some limitations. The small sample size due to the COVID-19 pandemic and the high dropout rate limited the generalizability of the results for the treatment and sham groups. Another limitation was the inability to assess long-term follow-up effects due to existing constraints to demonstrate the long-term effects after three and six months.
Conclusion
tDCS leads to improvement in motor learning, cognitive functions, and depressive symptoms in stroke patients.
Acknowledgment: The authors express their gratitude to all research participants and their families, as well as the respected staff of Valiasr Hospital in Zanjan City.
Ethical Permissions: This study was approved by the Ethics Committee of Zanjan University of Medical Sciences with the ethics code (IR.ZUMS.REC.1398.208).
Conflicts of Interests: The authors declare no conflicts of interest.
Authors' Contribution: Zakerian Zadeh A (First Author), Main Researcher/Discussion Writer/Statistical Analyst (30%); Dadashi M (Second Author), Methodologist (25%); Maghbooli M (Third Author), Assistant Researcher (15%); Zarei F (Fourth Author), Assistant Researcher (15%); Zakeryanzade R (Fifth Author), Introduction Writer/Assistant Researcher (15%)
Funding/Support: This article results from a Master's thesis by Seyed Ahmad Reza Zakarian Zadeh, which was financially supported by the Research and Technology Deputy of Zanjan University of Medical Sciences.
Stroke is increasingly prevalent in developing countries and is the second leading cause of death worldwide and the primary cause of disability [1]. Symptoms of stroke include various types of impairments such as motor, sensory, linguistic, and other cognitive functions [2]. Motor impairments following stroke are very common and are major contributors to disability post-stroke, also providing a basis for persistent neurobiological and cognitive sequelae [3].
Approximately 70-80% of stroke patients suffer from motor deficits, and a significant portion of them experience cognitive impairments related to stroke. Motor and cognitive deficits in stroke are interrelated with strong associations between activities such as walking, balance, attentional cognitive domains, and executive functioning [4]. About two-thirds of stroke patients experience cognitive impairments in the acute phase; about 30% of these patients recover spontaneously, and many are still at risk for cognitive impairment [5, 6]. According to recent research, post-stroke cognitive impairment in adults may result in white matter damage in brain regions distant from the primary lesion site. Changes in cognitive function over time after stroke and their association with the trajectory of white matter integrity in the brain provide a pathway for patient management and identification in the early stages [7].
Despite the favorable improvement in physical symptoms, 50% of stroke patients still suffer from cognitive impairment or limitations in rebuilding their normal lives, and 30% exhibit symptoms of depression 2 to 3 years after the stroke [8]. The prevalence of depression has been reported in 10% to 60% of studies. Depression is one of the most common psychological consequences of stroke, often accompanied by persistent disability, cognitive impairments, and mortality. Its onset typically occurs 6 to 24 months after the stroke, which may last up to 3 years in some patients [9]. Given the significant prevalence of stroke and the likelihood of depression in a large percentage of affected patients, efforts to improve and treat depression not only reduce the risk of subsequent strokes but also improve functionality, reduce mortality, and decrease the need for post-stroke healthcare [10]. The determinants of depression after stroke largely depend on the location and extent of the lesion, silent infarction, white matter damage, and brain atrophy [11]. However, no association has been observed between cerebral vascular lesions and depression indicative of functional brain disturbances due to structural brain damage [12]. Treatments mainly focus on rehabilitating motor deficits, as motor impairments are more unpleasant than cognitive disabilities [13].
Transcranial Direct Current Stimulation (tDCS) is a non-invasive and painless technique that induces cortical excitability [14]. Based on the evidence, tDCS is considered a safe method in human and animal studies and has no known irreversible damage [15]. Depression symptoms are usually associated with changes in cortical activity and excitability, especially in prefrontal regions [16]. They reflect an alteration in a distributed cortico-subcortical network across the hemispheres [17]. Therefore, it is reasonable to assume that modifying this pathological state with brain stimulation techniques may provide a therapeutic target. In addition to repetitive transcranial magnetic stimulation, transcranial direct current stimulation (tDCS) modulates cortical excitability for hours after stimulation cessation, making it a promising non-invasive therapeutic option [18]. Stimulation of the dorsolateral prefrontal cortex (DLPFC) is the most common site of brain cortex stimulation for reducing depression symptoms and improving mood and cognitive and attentional functions [19].
When standard anodal tDCS is used for stimulation, cortical function improves compared to baseline, while cathodal stimulation inhibits motor potentials, thereby implicitly enhancing motor learning processes [20]. Researchers believe that left dorsolateral prefrontal cortex (DLPFC) stimulation accounts for 23% of the changes in upper limb motor function, along with improvements in executive function, working memory, and attentional performance [21], suggesting that cognitive domains should not be evaluated independently as they influence each other, much like motor functions impacting attentional capacities [5].
Recent studies indicate that tDCS enhances motor functions in chronic stroke patients [22, 23]. Facilitation of motor learning through cortical excitability modulation induced by anodal tDCS may strengthen neuronal connectivity, thus improving motor learning processes [22]. Kang et al. confirm the long-term effects of motor learning in stroke patients treated with tDCS [24]. Yamaguchi et al. demonstrate that a combination of attention and tDCS is an effective strategy for enhancing rehabilitation in stroke patients with neurologic impairments [25]. The left DLPFC region integrates retained information in active memory with upcoming actions, where the left DLPFC is involved in encoding information. At the same time, the right DLPFC is engaged in information retrieval [26]. Although the increase in activity in the DLPFC during planning a complex task aligns with the idea that the DLPFC links information (a visual cue) to future action (required movement), the impact of tDCS on the DLPFC has only been investigated in cognitive tasks such as active memory and executive function [27]. At the same time, its effect on motor behavior remains largely unknown [28].
Although non-invasive stimulation techniques like tDCS are recognized as potential, innovative, cost-effective, portable, and easily manageable therapeutic methods for improving both motor and cognitive abilities, there are still unknown factors. Customized protocols involving consecutive stimulation and inhibition of the motor cortex (M1 and M2) and stimulation and inhibition of the left and right DLPFC hemispheres have not yet been utilized in studies, and sufficient data on stimulating two brain regions simultaneously are lacking. Therefore, given the high prevalence of stroke-related complications such as motor and cognitive impairments and depression in ischemic stroke patients, the present study aimed to determine the effect of transcranial direct current stimulation (tDCS) on depression symptoms, motor learning, and cognitive function in ischemic stroke patients.
Materials and Methods
This randomized controlled clinical trial by transcranial direct current stimulation (tDCS) was conducted on all patients with ischemic stroke referred to Vali-Asr University Hospital in Zanjan City, Iran, during 2019-2020. According to Delavar, in experimental research, a minimum sample size of 15 individuals per group is required under controlled conditions [29]. A sample size of 15 individuals per group was considered. 70 patients were initially introduced to participate. Due to the COVID-19 pandemic, only 35 patients between 18 and 85 with a diagnosis of chronic ischemic stroke confirmed by imaging, basic education, right-hand, at least 3 months elapsed since the onset of the infarction, presence of a deficit based on measurement tools, and mild to severe depressive symptoms based on the Hamilton Depression Rating Scalemet were enrolled in the study. Individuals with suicidal ideation or risks that prevent maintaining drug dosage, psychiatric disorders, substance abuse, history of head injury and epileptic seizures, contraindications to tDCS use, seizures, and presence of a cardiac pacemaker were not entered. They were randomly allocated to three groups (11 patients in the tDCS group, 12 patients in the Sham group, and 12 patients in the control group) using the Random Number Generator v1.3 web-based program. A total of 11 patients withdrew from the study due to various reasons, such as unwillingness to undergo an MRI for diagnosis before the study, fear of the COVID-19 pandemic, consecutive absences from two intervention sessions, difficulty in mobility due to severe motor impairment, etc. Eventually, 24 patients with ischemic stroke remained (Figure 1).

Figure 1. Consort diagram of participants
Data Collection
The Fugl-Meyer test, Hamilton Depression Rating Scale, Montreal Cognitive Assessment (MCA), and Mini-Mental State Examination (MMSE) were used to assess motor learning, depression, and cognitive function, respectively.
The Fugl-Meyer Test was utilized to evaluate upper and lower extremity motor performance. The test items included 50 movements categorized into 6 levels of improvement, with 33 items related to the upper extremity in the shoulder, elbow, forearm, wrist, and hand. Each item is scored from 0 to 2. 0: if the individual is unable to perform the movement, 1: if the movement is incomplete, and 2: if the movement is fully performed. The total score for the upper extremity is 66 [30-32]. The reliability between the two examiners in this test in the study by Duncan & Stanford was 0.97 and 0.96, respectively [33]. In the study by Karimi et al., the reliability between two examiners was 0.977 [34].
The Hamilton Depression Rating Scale was used to measure depression in participants. This scale consists of 21 items. Higher scores indicate more severe depressive symptoms, with participant responses to questions classified as follows: 0-7 (no depression), 8-17 (mild depression), 18-24 (moderate depression), and above 25 (severe depression). The reliability and validity of this test have been reported as 0.90 [35]. The reliability (Cronbach's alpha) of this test was 0.88.
For cognitive performance evaluation, the Montreal Cognitive Assessment (MCA) and the Mini-Mental State Examination (MMSE) were used. The MCA is a cognitive screening questionnaire designed by Julayanont et al. [36] to detect mild cognitive impairment. The purpose of administering this test was to overcome the limitations of the MMSE (Mini-Mental State Examination). This test assesses 8 cognitive domains through various skills. The maximum score for this test is 30, and the cutoff point is set at 24. The test is completed in less than 15 minutes. For individuals with less than 12 years of education, 1 point is added to their total score. The reliability of this test, based on Cronbach's alpha, was 92%. In Iran, the Montreal Cognitive Assessment scale has been validated by Seigaroody et al. [37].
Procedure
After being registered on IRCT (Code 42561), and the definitive diagnosis of stroke by a neurology specialist and clinical psychology interview with the patient, the Fugl-Meyer Test for motor learning evaluation, the Hamilton Depression Rating Scale for depression measurement, and cognitive assessment tests including MoCA and MMSE for cognitive function assessment were done by the patient on paper. Additionally, brain imaging was performed with MRI to record the stroke status and diagnose the disease by a neurologist.
This study included three groups, and the intervention implementation for each group was as follows:
1- First group (real intervention group): Anodal stimulation was performed consecutively in the primary motor areas affected and the left dorsolateral prefrontal cortex (M1damH+Left-DLPFC), with 20 minutes of anodal stimulation to M1damH, followed by 30 minutes of rest without stimulation, and finally, 20 minutes of anodal stimulation to Left-DLPFC using the tDCS device. The tDCS intervention was conducted for a total of 12 sessions, one per day.
2- Second group (placebo intervention group): Anodal stimulation was performed in the primary motor areas affected and left dorsolateral prefrontal cortex (M1damH+Left-DLPFC). The anodal stimulation to M1damH was discontinued one minute after the start of stimulation, followed by 30 minutes of rest, and finally, anodal stimulation to Left-DLPFC, which was discontinued one minute after the start of stimulation. The stimulation was performed using the tDCS device.
3- Third group (control group): This group received only routine treatment.
In addition to the research interventions, the first and second groups also received routine treatment.
To assess the study's outcomes, questionnaires were completed after the sessions and one month later to evaluate motor learning, depressive symptoms, and cognitive function. The patients were unaware of the group they were assigned to and the type of intervention. Pre-test and post-test evaluations of the patients were performed by another individual other than the therapist.
Statistical analysis
The data were analyzed using descriptive statistics (mean, standard deviation, frequency, and tables) and inferential statistics (multivariate analysis of covariance) with the necessary assumptions using SPSS 23 software.
Findings
The mean age of the patients was 64.12±14.8 years (p=0.062), and the mean time since stroke was 47.60±38.99 months (p=0.437). There were no significant differences in demographic characteristics between the study groups (Table 1).
Table 1. Comparing the frequency of demographic characteristics between the study groups

The assumption of homogeneity of variances was met, and there was no obstacle to performing a multivariate analysis of covariance. Based on the multivariate analysis using Wilks’, tDCS and Sham interventions significantly affected the study groups regarding motor performance, depressive symptoms, and cognitive function, as measured by MMSE and MOCA (p<0.05). The T-test showed a significant effect of tDCS on post-test and follow-up motor performance, depressive symptoms, and MMSE, MoCA cognitive function in the tDCS intervention group (p<0.05). At the same time, it was not significant in the Sham and Control groups (Table 2).
Table 2. Wilks' Lambda multivariate test for research variables (df=4; err. df=38)

There was a significant difference between the study groups in motor learning, depression, and cognitive performance assessed at post-intervention and 1-month follow-up (p<0.001; Table 3). The effect size ranged from 30 to 63%, indicating a substantial impact of the intervention and improvement in post-intervention and follow-up assessments (Figure 2).
Table 3. Results of multivariate analysis of covariance between groups in post-test and follow-up (df=2)



Figure 2. Comparing the score of (a) motor performance in the upper limbs, (b) motor performance in the lower limbs, (c) Hamilton depression, (d) Mini-Mental State Examination (MMSE), and (e) Montreal Cognitive Assessment (MCA)
There were significant differences in cognitive performance, depressive symptoms, and MoCA cognitive function between the tDCS and the Sham and Control groups in the post-test and follow-up (p<0.05). Similarly, there was no significant difference between the Sham and Control groups in cognitive performance, depressive symptoms, and MMSE and MoCA cognitive function in the post-intervention and follow-up assessments (Table 4).
Table 4. Comparing between tDCS and Sham and control (using the Bonferroni follow-up test) at post-intervention and follow-up

Discussion
This study aimed to evaluate depression symptoms, motor learning, and cognitive function after transcranial direct current stimulation (tDCS) in ischemic stroke patients. The novelty of this research is the simultaneous anodal stimulation of two areas. The results indicated that tDCS improved depression symptoms, motor function, and cognitive function in patients with stroke, while no significant improvement was observed in the sham and control groups.
In this study, tDCS improved motor learning. Similarly, using a systematic review and meta-analysis, Šimko et al. have shown that anodal stimulation in the damaged hemisphere and cathodal inhibition in the contralateral hemisphere led to long-term improvement in motor learning with tDCS [21]. Additionally, Yamaguchi et al. have found that tDCS enhances motor learning in healthy individuals by stimulating the brain cortex [25]. In accordance with this study, Elsner et al. have demonstrated that tDCS improves motor function in stroke patients [38, 39]. Oveisgharan et al. have reported that left DLPFC stimulation combined with M1 stimulation resulted in greater improvement in motor function compared to M1 stimulation alone, with left DLPFC stimulation being safe and effective in improving hand paralysis after acute stroke [40]. Furthermore, Elsner et al. have shown improvement in upper and lower limb function, muscle strength, and cognitive ability with tDCS after stroke, highlighting its importance [38].
The present study differs from previous ones in the number of sessions and the intervention protocol (sequential anodal stimulation of M1 and DLPFC in the affected hemisphere). Previous interventions focused on anodal stimulation in a single brain region (M1 or DLPFC), whereas this study compared three groups: Active stimulation, Sham, and control, which were not previously examined.
Kang et al. [24] stated that each of the three tDCS protocols (anodal stimulation on M1 in the affected hemisphere, cathodal stimulation on M1 in the unaffected hemisphere, and bilateral stimulation on both hemispheres) produces long-term learning effects, significantly improving stroke patients' recovery. It has been shown that brain stimulation and rehabilitation can increase upper limb improvement by 20% in the intervention group compared to the control, which were statistically significant [41]. This suggests that tDCS could be an effective intervention alongside other rehabilitation methods for improving movement in stroke patients [37, 38].
A-tDCS enhances cortical excitability under all conditions, immediately after stimulation; Therefore, the efficacy of A-tDCS depends on the current density and duration of use [25]. A-tDCS increases cortical excitability in both healthy individuals and stroke patients, favoring improved motor function following A-tDCS [42]. The neural circuits associated with ankle tracking during motor tasks are likely activated or enhanced, which may facilitate synaptic plasticity during tDCS, resulting in improvement [43]. Motor learning usually involves activity-dependent synaptic changes, leading to neural plasticity such as LTP-like or LTD-like changes in cortical neurons [43], which can sustain improvement after intervention cessation.
Cognitive function in stroke patients improved after tDCS intervention. Similarly, Berryhill & Martin have demonstrated that tDCS creates stable and long-term cognitive effects in clinical and healthy samples [44].
Furthermore, depression symptoms improved in patients after the tDCS intervention. Consistent with the present findings, Shiozawa et al. have shown that brain electrical stimulation can reduce depressive symptoms [45].
It can be suggested that the observed effects may result from increased cortical excitability in the left lateral prefrontal cortex, as anodal stimulation depolarizes neurons, leading to changes in neuronal resting state and excitability in that area [46]. This stimulation enhances the networks involved in various cognitive tasks. Another hypothesis could involve the role of dopamine in memory and cognitive tasks. Increased excitability in the superficial layers of the prefrontal cortex may lead to increased dopamine release, which in turn may improve cognitive performance and problem-solving. Additionally, constant electrical flow can lead to changes in local ion concentration, affecting membrane-permeable proteins and hydrogen ions (H+) [47], contributing to improved excitability. Another hypothesis could involve activating other brain regions by stimulating the lateral posterior prefrontal cortex, which may improve short-term memory, visuospatial skills, executive functions, attention, concentration, working memory, language, and awareness of time and place in stroke patients [48].
Another interpretation regarding the efficacy of tDCS on cognitive functions in the follow-up evaluation showed that the improvement in cognitive functions compared to the post-test decreased. Considering that cognitive functions are a complex and widespread capability throughout the brain, particularly involving the prefrontal areas, it seems that increasing the number of tDCS stimulation sessions or cyclically administering tDCS after the completion of the initial ten-session period may help to sustain the effects of cognitive function change and improvement over time.
To sum up, brain electrical stimulation is a suitable and cost-effective intervention for improving cognitive functions and motor learning, and reducing depressive symptoms after stroke can be beneficial. Therefore, for cognitive rehabilitation and prevention of disorders in healthcare and counseling centers, it is necessary to consider this type of intervention as a safe method, in addition to pharmacological treatment and other rehabilitation methods for stroke patients.
This study had some limitations. The small sample size due to the COVID-19 pandemic and the high dropout rate limited the generalizability of the results for the treatment and sham groups. Another limitation was the inability to assess long-term follow-up effects due to existing constraints to demonstrate the long-term effects after three and six months.
Conclusion
tDCS leads to improvement in motor learning, cognitive functions, and depressive symptoms in stroke patients.
Acknowledgment: The authors express their gratitude to all research participants and their families, as well as the respected staff of Valiasr Hospital in Zanjan City.
Ethical Permissions: This study was approved by the Ethics Committee of Zanjan University of Medical Sciences with the ethics code (IR.ZUMS.REC.1398.208).
Conflicts of Interests: The authors declare no conflicts of interest.
Authors' Contribution: Zakerian Zadeh A (First Author), Main Researcher/Discussion Writer/Statistical Analyst (30%); Dadashi M (Second Author), Methodologist (25%); Maghbooli M (Third Author), Assistant Researcher (15%); Zarei F (Fourth Author), Assistant Researcher (15%); Zakeryanzade R (Fifth Author), Introduction Writer/Assistant Researcher (15%)
Funding/Support: This article results from a Master's thesis by Seyed Ahmad Reza Zakarian Zadeh, which was financially supported by the Research and Technology Deputy of Zanjan University of Medical Sciences.
Keywords:
References
1. Campbell BCV, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, et al. Ischaemic stroke. Nat Rev Dis Primers. 2019;5:70. [Link] [DOI:10.1038/s41572-019-0118-8]
2. Shajahan S, Sun L, Harris K, Wang X, Sandset EC, Yu AY, et al. Sex differences in the symptom presentation of stroke: A systematic review and meta-analysis. Int J Stroke. 2023;18(2):144-53. [Link] [DOI:10.1177/17474930221090133]
3. Al-Hussain F, Nasim E, Iqbal M, Altwaijri N, Asim N, Yoo WK, et al. The effect of transcranial direct current stimulation combined with functional task training on motor recovery in stroke patients. Medicine. 2021;100(6):e24718. [Link] [DOI:10.1097/MD.0000000000024718]
4. Verstraeten S, Mark R, Sitskoorn M. Motor and cognitive impairment after stroke: A common bond or a simultaneous deficit?. Stroke Res Ther. 2016;1(1). [Link]
5. Draaisma LR, Wessel MJ, Hummel FC. Non-invasive brain stimulation to enhance cognitive rehabilitation after stroke. Neurosci Lett. 2020;719:133678. [Link] [DOI:10.1016/j.neulet.2018.06.047]
6. Chi X, Wang L, Liu H, Zhang Y, Shen W. Post-stroke cognitive impairment and synaptic plasticity: A review about the mechanisms and Chinese herbal drugs strategies. Front Neurosci. 2023;17:1123817. [Link] [DOI:10.3389/fnins.2023.1123817]
7. Wood H. Remote white matter integrity influences cognitive function after stroke. Nat Rev Neurol. 2016;12:616. [Link] [DOI:10.1038/nrneurol.2016.148]
8. Kapoor A, Lanctôt KL, Bayley M, Kiss A, Herrmann N, Murray BJ, et al. "Good outcome" isn't good enough: Cognitive impairment, depressive symptoms, and social restrictions in physically recovered stroke patients. Stroke. 2017;48(6):1688-90. [Link] [DOI:10.1161/STROKEAHA.117.016728]
9. Robinson RG. Poststroke depression: Prevalence, diagnosis, treatment, and disease progression. Biol Psychiatry. 2003;54(3):376-87. [Link] [DOI:10.1016/S0006-3223(03)00423-2]
10. Williams LS. Depression and stroke: Cause or consequence?. Semin Neurol. 2005;25(4):396-409. [Link] [DOI:10.1055/s-2005-923534]
11. Vataja R, Pohjasvaara T, Leppävuori A, Mäntylä R, Aronen HJ, Salonen O, et al. Magnetic resonance imaging correlates of depression after ischemic stroke. Arch Gen Psychiatry. 2001;58(10):925-31. [Link] [DOI:10.1001/archpsyc.58.10.925]
12. Lassalle-Lagadec S, Sibon I, Dilharreguy B, Renou P, Fleury O, Allard M. Subacute default mode network dysfunction in the prediction of post-stroke depression severity. Radiology. 2012;264(1):218-24. [Link] [DOI:10.1148/radiol.12111718]
13. Sun JH, Tan L, Yu JT. Post-stroke cognitive impairment: Epidemiology, mechanisms and management. Ann Transl Med. 2014;2(8):80. [Link]
14. Vergallito A, Varoli E, Pisoni A, Mattavelli G, Del Mauro L, Feroldi S, et al. State-dependent effectiveness of cathodal transcranial direct current stimulation on cortical excitability. NeuroImage. 2023;277:120242. [Link] [DOI:10.1016/j.neuroimage.2023.120242]
15. Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. Safety of transcranial direct current stimulation: Evidence based update 2016. Brain Stimul. 2016;9(5):641-61. [Link] [DOI:10.1016/j.brs.2016.06.004]
16. Razza LB, Palumbo P, Moffa AH, Carvalho AF, Solmi M, Loo CK, et al. A systematic review and meta‐analysis on the effects of transcranial direct current stimulation in depressive episodes. Depress Anxiety. 2020;37(7):594-608. [Link] [DOI:10.1002/da.23004]
17. Suen PJ, Doll S, Batistuzzo MC, Busatto G, Razza LB, Padberg F, et al. Association between tDCS computational modeling and clinical outcomes in depression: Data from the ELECT-TDCS trial. Eur Arch Psychiatry Clin Neurosci. 2021;271(1):101-10. [Link] [DOI:10.1007/s00406-020-01127-w]
18. Jog MA, Anderson C, Kubicki A, Boucher M, Leaver A, Hellemann G, et al. Transcranial direct current stimulation (tDCS) in depression induces structural plasticity. Sci Rep. 2023;13:2841. [Link] [DOI:10.1038/s41598-023-29792-6]
19. Kaminski E, Carius D, Knieke J, Mizuguchi N, Ragert P. Complex sequential learning is not facilitated by transcranial direct current stimulation over DLPFC or M1. Eur J Neurosci. 2024. [Link] [DOI:10.1111/ejn.16255]
20. Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57(10):1899-901. [Link] [DOI:10.1212/WNL.57.10.1899]
21. Šimko P, Pupíková M, Gajdoš M, Klobušiaková P, Vávra V, Šimo A, et al. Exploring the impact of intensified multiple session tDCS over the left DLPFC on brain function in MCI: A randomized control trial. Sci Rep. 2024;14:1512. [Link] [DOI:10.1038/s41598-024-51690-8]
22. Navarro‐López V, Del‐Valle‐Gratacós M, Carratalá‐Tejada M, Cuesta-Gómez A, Fernández-Vázquez D, Molina‐Rueda F. The efficacy of transcranial direct current stimulation (tDCS) on upper extremity motor function after stroke: A systematic review and comparative meta‐analysis of different stimulation polarities. PM R. 2023. [Link] [DOI:10.1002/pmrj.13088]
23. Tedla JS, Sangadala DR, Reddy RS, Gular K, Kakaraparthi VN, Asiri F. Transcranial direct current stimulation (tDCS) effects on upper limb motor function in stroke: An overview review of the systematic reviews. Brain Inj. 2023;37(2):122-33. [Link] [DOI:10.1080/02699052.2022.2163289]
24. Kang N, Summers JJ, Cauraugh JH. Transcranial direct current stimulation facilitates motor learning post-stroke: A systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2016;87(4):345-55. [Link] [DOI:10.1136/jnnp-2015-311242]
25. Yamaguchi T, Moriya K, Tanabe S, Kondo K, Otaka Y, Tanaka S. Transcranial direct-current stimulation combined with attention increases cortical excitability and improves motor learning in healthy volunteers. J Neuroeng Rehabil. 2020;17:23. [Link] [DOI:10.1186/s12984-020-00665-7]
26. Adkins TJ, Lee TG. Reward modulates cortical representations of action. NeuroImage. 2021;228:117708. [Link] [DOI:10.1016/j.neuroimage.2020.117708]
27. Brunoni AR, Vanderhasselt MA. Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: A systematic review and meta-analysis. Brain Cogn. 2014;86:1-9. [Link] [DOI:10.1016/j.bandc.2014.01.008]
28. Fujiyama H, Van Soom J, Rens G, Cuypers K, Heise KF, Levin O, et al. Performing two different actions simultaneously: The critical role of interhemispheric interactions during the preparation of bimanual movement. Cortex. 2016;77:141-54. [Link] [DOI:10.1016/j.cortex.2016.02.007]
29. Delavar A. Theoretical and practical research in the humanities and social sciences. Tehran: Roshd Publication; 2015. [Link]
30. Rabadi M, Aston C. Effect of transcranial direct current stimulation on severely affected arm-hand motor function in patients after an acute ischemic stroke (667). Neurology. 2020;94(15). [Link] [DOI:10.1212/WNL.94.15_supplement.667]
31. Dukas R. Evolutionary biology of animal cognition. Annu Rev Ecol Evol Syst. 2004;35:347-74. [Link] [DOI:10.1146/annurev.ecolsys.35.112202.130152]
32. Nejati V. Cognitive abilities questionnaire: Development and evaluation of psychometric properties. Adv Cogn Sci. 2013;15(2):11-9. [Persian] [Link]
33. Sanford J, Moreland J, Swanson LR, Stratford PW, Gowland C. Reliability of the Fugl-Meyer assessment for testing motor performance in patients following stroke. Phys Ther. 1993;73(7):447-54. [Link] [DOI:10.1093/ptj/73.7.447]
34. Karimi E, Kalantary M, Shafiee Z, Tabatabaiee SM. Inter-rater reliability of the action research arm test & upper limb related fugel meyer test in adult with CVA from Qazvin. J Res Rehabil Sci. 2014;10(1):67-76. [Persian] [Link]
35. Reynolds WM, Kobak KA. Reliability and validity of the Hamilton Depression Inventory: A paper-and-pencil version of the Hamilton Depression Rating Scale Clinical Interview. Psychol Assess. 1995;7(4):472-83. [Link] [DOI:10.1037/1040-3590.7.4.472]
36. Julayanont P, Nasreddine ZS. Montreal Cognitive Assessment (MoCA): Concept and clinical review. In: Larner AJ, editor. Cognitive screening instruments. Cham: Springer; 2017. p. 139-95. [Link] [DOI:10.1007/978-3-319-44775-9_7]
37. Sikaroodi H, Majidi A, Samadi S, Shirzad H, Aghdam H, Azimi Kia A, et al. Evaluating reliability of the montreal cognitive assessment test and its agreement with neurologist diagnosed among patients with cognitive complaints. J Police Med. 2012;1(1):11-7. [Persian] [Link]
38. Elsner B, Kugler J, Pohl M, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving activities of daily living, and physical and cognitive functioning, in people after stroke. Cochrane Database Syst Rev. 2016;3(3):CD009645. [Link] [DOI:10.1002/14651858.CD009645.pub3]
39. Elsner B, Kwakkel G, Kugler J, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving capacity in activities and arm function after stroke: A network meta-analysis of randomized controlled trials. J Neuroeng Rehabil. 2017;14:95. [Link] [DOI:10.1186/s12984-017-0301-7]
40. Oveisgharan S, Organji H, Ghorbani A. Enhancement of motor recovery through left dorsolateral prefrontal cortex stimulation after acute ischemic stroke. J Stroke Cerebrovasc Dis. 2018;27(1):185-91. [Link] [DOI:10.1016/j.jstrokecerebrovasdis.2017.08.026]
41. Harvey RL, Winstein CJ, Everest Trial Group. Design for the everest randomized trial of cortical stimulation and rehabilitation for arm function following stroke. Neurorehabil Neural Repair. 2009;23(1):32-44. [Link] [DOI:10.1177/1545968308317532]
42. Bastani A, Jaberzadeh S. Does anodal transcranial direct current stimulation enhance excitability of the motor cortex and motor function in healthy individuals and subjects with stroke: A systematic review and meta-analysis. Clin Neurophysiol. 2012;123(4):644-57. [Link] [DOI:10.1016/j.clinph.2011.08.029]
43. Sriraman A, Oishi T, Madhavan S. Timing-dependent priming effects of tDCS on ankle motor skill learning. Brain Res. 2014;1581:23-9. [Link] [DOI:10.1016/j.brainres.2014.07.021]
44. Berryhill ME, Martin D. Cognitive effects of transcranial direct current stimulation in healthy and clinical populations: An overview. J ECT. 2018;34(3):e25-35. [Link] [DOI:10.1097/YCT.0000000000000534]
45. Shiozawa P, Cordeiro Q, Cho HJ, Trevizol AP, Brietzke E. A critical review of trials of transcranial direct current stimulation and trigeminal nerve stimulation for depression: The issue of treatment-emergent mania. Trends Psychiatry Psychother. 2017;39(1):48-53. [Link] [DOI:10.1590/2237-6089-2016-0027]
46. Marshall L, Mölle M, Siebner HR, Born J. Bifrontal transcranial direct current stimulation slows reaction time in a working memory task. BMC Neurosci. 2005;6:23. [Link] [DOI:10.1186/1471-2202-6-23]
47. Barbey AK, Koenigs M, Grafman J. Dorsolateral prefrontal contributions to human working memory. Cortex. 2013;49(5):1195-205. [Link] [DOI:10.1016/j.cortex.2012.05.022]
48. Javadi AH, Cheng P. Transcranial direct current stimulation (tDCS) enhances reconsolidation of long-term memory. Brain Stimul. 2013;6(4):668-74. [Link] [DOI:10.1016/j.brs.2012.10.007]









